Hybridoma cell strain capable of secreting anti-rift valley fever virus NSs protein monoclonal antibody and application thereof
A hybridoma cell line, monoclonal antibody technology, applied in antiviral immunoglobulin, analytical materials, biological material analysis, etc., can solve the problem of inability to distinguish infection antibodies and immune antibodies, and achieve good specificity and repeatability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation of embodiment 1 recombinant protein
[0022] 1. Construction of recombinant plasmids and protein expression
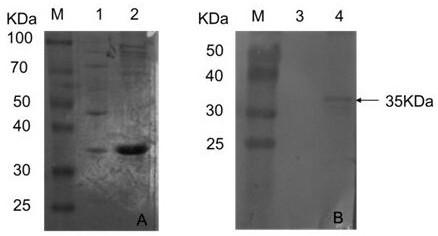
[0023] According to the Rift Valley fever virus NSs gene sequence (HE687307) published by Genbank, the sequence shown in SEQ ID NO:1 was obtained. The sequence shown in SEQ ID NO:1 was synthesized and cloned into pET-28a(+) to obtain the recombinant plasmid pET-28a-NSs. The recombinant plasmid pET-28a-NSs was transformed into Escherichia coli competent cells, and the positive colonies were picked and named pET-28a-NSs (BL21) after PCR identification. Inoculate pET-28a-NSs (BL21) into LB liquid medium containing kanamycin for culture, when OD 600 When it reaches 0.6-0.8, add 0.5mmol / L IPTG, induce expression at 37°C for 5h, collect the cells and lyse them with ultrasonic waves, collect the supernatant and precipitate of the lysate, and identify the expression of the recombinant protein by SDS-PAGE gel electrophoresis. Transform pET-28a(+) into ...
Embodiment 2
[0028] Embodiment 2 Establishment of Monoclonal Antibody Hybridoma Cell Line
[0029] 1. Immunization of BALB / c mice
[0030] 100 μg of recombinant NSs protein and Freund's adjuvant were mixed and emulsified at a volume ratio of 1:1, and then subcutaneously injected into BALB / c mice (100 μg / mouse). Afterwards, two booster immunizations were performed, with an interval of two weeks between each time and the previous immunization. For each booster immunization, a mixture of 100 μg of recombinant NSs protein and incomplete Freund's adjuvant was mixed and emulsified at a ratio of 1:1. A total of 3 times of immunization. Blood was collected 2 weeks after the third immunization, and the serum titer of immunized mice was detected by indirect ELISA. The indirect ELISA method is as follows: the recombinant NSs protein was coated with a concentration of 2 μg / mL on a 96-well microtiter plate, 100 μL / well, overnight at 4°C. Wash with PBST 3 times and pat dry; add PBST containing 0.5% B...
Embodiment 3
[0040] Example 3 Establishment of RVFV NSs Blocking ELISA Antibody Detection Method
[0041] 1. Determination of the coating concentration of the antigen and the dilution of the enzyme-labeled monoclonal antibody
[0042] The recombinant NSs protein was diluted to 0.5 μg / mL, 1 μg / mL, 2 μg / mL, 4 μg / mL with antigen coating solution (0.05 mol / L, pH 9.6 carbonate buffer) by square array titration method, Add 100 μL to each well, incubate overnight at 4°C; wash three times with PBST (PBS containing 0.5% Tween-20); add 300 μL PBST containing 0.5% BSA to each well, block at 37°C for 2 hours; wash three times with PBST; Add 1:10 diluted RVFV negative and positive sera to the antigen concentration, incubate at 37°C for 1 hour; wash with PBST 3 times; add 100 μL respectively and use PBST according to the dilution of 1:500, 1:1000, 1:1500, 1:2000, 1 :2500, 1:3000 diluted HRP-2D6D10 (the concentration of HRP-2D6D10 before dilution was 1mg / mL), incubated at 37°C for 1h; washed 3 times wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com