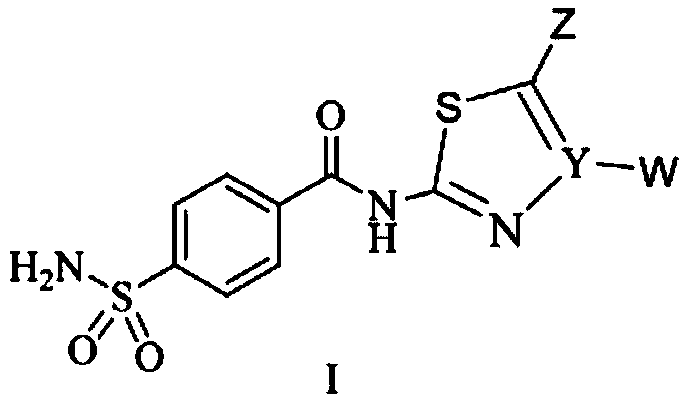
Benzenesulfonamide compound containing five-membered heterocycle and preparation method and application thereof
A technology of benzenesulfonamide and five-membered heterocyclic ring, which is applied in the field of pharmacy and can solve problems such as poor water solubility, limited application, and obvious side effects of acetazolamide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
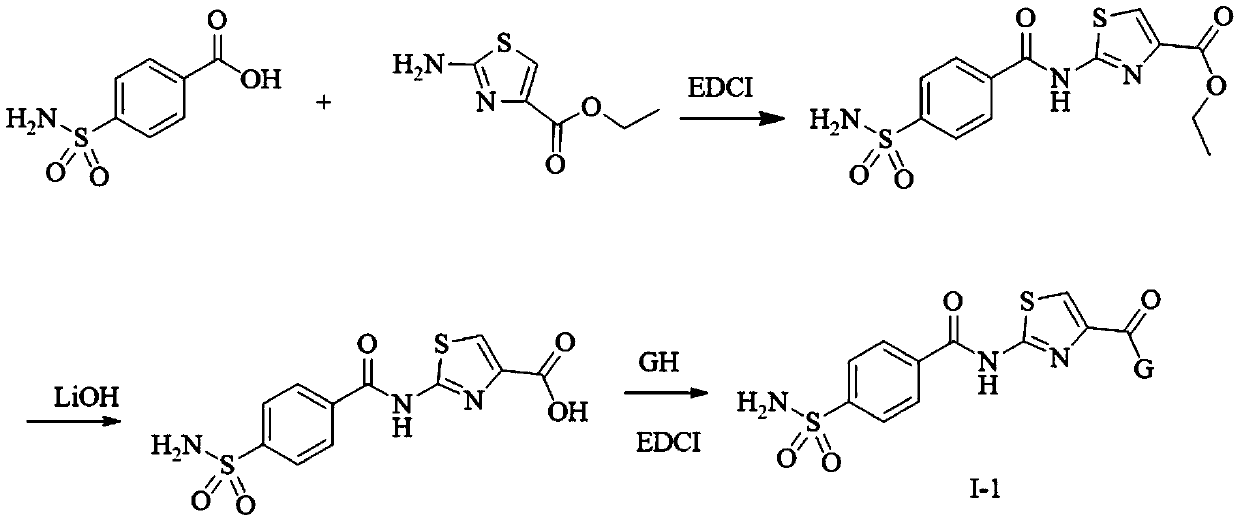
[0142] Example 1: Ethyl 2-[(4-sulfamoylphenyl)formyl]aminothiazole-4-carboxylate
[0143]
[0144] 4-carboxybenzenesulfonamide (10.0mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI, 12.0mmol) and 1-hydroxybenzotriazole ( HOBT, 12.0 mmol) was added to DMF (10 ml) and stirred at room temperature for 30 minutes. Then 2-amino-4-ethoxycarbonylthiazole (12.0 mmol) and DMAP (3.0 mmol) were added. React at 45°C until the reaction is complete as detected by TLC. The mixture was cooled to room temperature and extracted with ethyl acetate. The organic layer was washed with brine, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The starting product was purified by column chromatography (DCM / MT60:1-30:1) to obtain a white solid compound with a yield of 69%. 1H NMR (DMSO-d6) δppm: 13.30 (s, 1H, CONH), 8.27 (d, J = 8.0Hz, 2H, Ar-H), 8.18 (s, 1H, S-CH), 7.98 (d, J =8.0Hz,2H,Ar-H),7.60(s,2H,SO 2 NH 2 ),4.32(q,2H,OCH 2 )...
Embodiment 2
[0145] Example 2: Ethyl 5-[(4-sulfamoylphenyl)formyl]amino-1,3,4-thiadiazole-2-carboxylate
[0146]
[0147] Except for replacing the corresponding reaction starting materials, the target compound was prepared according to the method of Example 1, a white solid, and the yield was 73%. 1 H NMR (DMSO-d6) δppm: 13.77 (s, 1H, CONH), 8.29 (d, J = 8.0Hz, 2H, Ar-H), 8.00 (d, J = 8.0Hz, 2H, Ar-H), 7.61(s,2H,SO 2 NH 2 ),4.43(q,2H,OCH 2 ), 1.37(t,3H,CH 3 ); 13 CNMR(DMSO-d6)δppm: 165.42, 163.26, 159.50, 154.57, 148.38, 134.52, 129.93, 126.41, 63.02, 14.53; ESI-MS: 357.01[M+H] + .
Embodiment 3
[0148] Example 3: N-cyclopropyl-2-[(4-sulfamoylphenyl)formyl]aminothiazole-4-carboxamide
[0149]
[0150] The first step: the preparation of 2-(4-sulfamoylbenzamido)thiazole-4-carboxylic acid
[0151]
[0152] Ethyl 2-(4-sulfamoylbenzamido)thiazole-4-carboxylate (3.56 mmol) was dissolved in tetrahydrofuran (THF, 15 ml). Lithium hydroxide (10.68 mmol) was then added, and the reaction was stirred at room temperature. TLC detected that the reaction was complete, and the pH value of the mixture was adjusted to 5-6 with 10% aqueous hydrochloric acid. After filtering and washing the filter cake with methanol, a white solid compound was obtained with a yield of 91%. 1 H NMR (DMSO-d6) δppm: 13.20-13.00 (m, 2H, CONH, COOH), 8.27 (d, J = 8.0H Z ,2H,Ar-H),8.10(s,1H,CH),7.98(d,J=8.0H Z ,2H,Ar-H),7.59(s,2H,SO 2 NH 2 ); ESI-MS: 328.00[M+H] + .
[0153] The second step: the preparation of N-cyclopropyl-2-(4-sulfamoylbenzamido)thiazole-4-carboxamide
[0154]
[0155] 2-(4-Su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com