Cell surface glycan in-situ electrogenerated fluorescence imaging analysis method based on a bipolar electrode
A cell surface and fluorescence imaging technology, applied in the field of biosensing, can solve the problems of insufficient sensitivity, difficult to obtain raw materials, difficult to analyze glycans, etc., and achieve the effects of avoiding mutual interference, reducing consumption and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the synthesis of nanoprobe
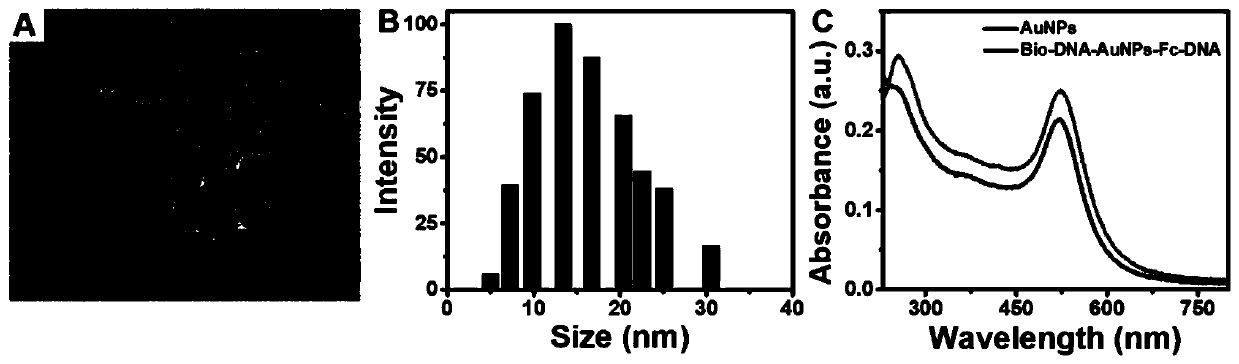
[0041] The specific steps for the synthesis of 13nm AuNPs are as follows: the magnetron, two-neck flask and condenser tube used in the experiment were soaked in an alkali tank overnight, soaked in aqua regia for 24 hours, and then rinsed with a large amount of primary water and secondary water before use. to boiling HAuCl 4 solution (1 mM, 50 mL), quickly added trisodium citrate solution (38.8 mM, 5 mL), stirred continuously and kept boiling for 15 minutes, while the color of the solution changed from clear to black, purple, and finally to Deep red, the solution was cooled to room temperature. Finally, the prepared AuNPs solution was stored at 4 °C for later use.
[0042] The specific steps of Bio-DNA-AuNPs-Fc-DNA synthesis were as follows: 1 mg BSPP was added to the above-prepared AuNPs aqueous solution (10 nM, 1.5 mL) to replace the citrate ligand to protect AuNPs. To increase the stability of AuNPs-DNA complexes, incubate...
Embodiment 2
[0046] Example 2: Principle Verification 1
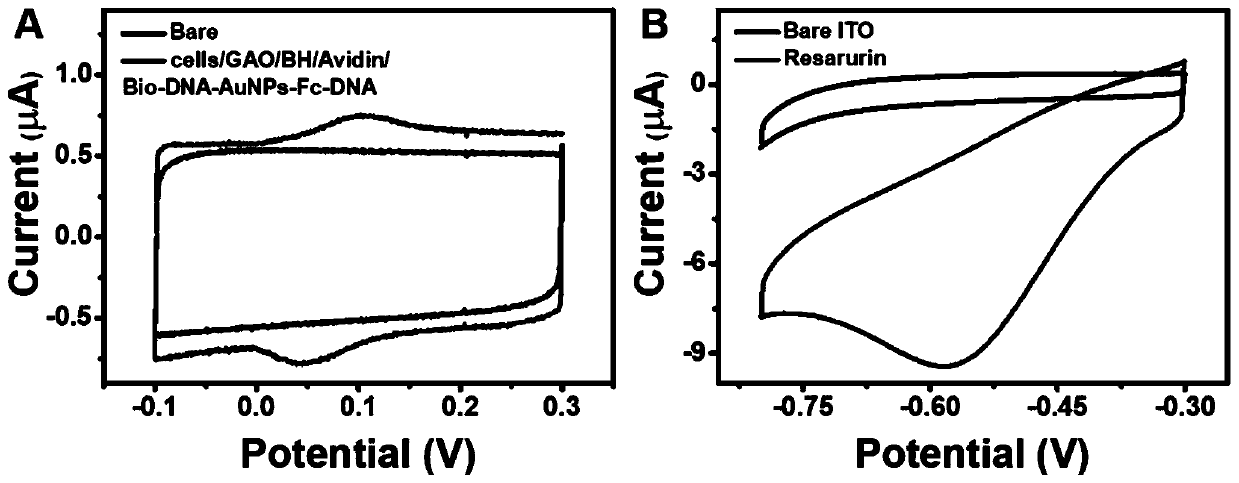
[0047]1) We first measured the cyclic voltammetry curve of the ITO electrode of the cultured cells in 0.1 M PBS solution, and no obvious redox peak appeared; -1 GAO, 10 mM aniline, a mixture of 100 μM BH and 5% FBS, 0.1 mg mL −1 The CV curves of avidin and 4 nM Bio-DNA-AuNPs-Fc-DNA, the CV curves of the gradually modified electrode showed obvious redox peaks at 0.106 V and 0.045 V, and the detection results showed that using this method, the electrode The active molecule Fc can be successfully modified, see image 3 a.
[0048] 2) Secondly, the saturation N of the ITO electrode was determined 2 In the cyclic voltammetry curves of 0.1 M PBS solution and 50 μM resazurin in the PBS solution in the atmosphere, there is no obvious redox peak in the CV curve containing only PBS, but it can be seen in the CV curve of resazurin Resazurin reduction peak appeared at -0.579 V. Experimental results show that resazurin can be reduced.
Embodiment 3
[0049] Example 3: Principle Verification 2
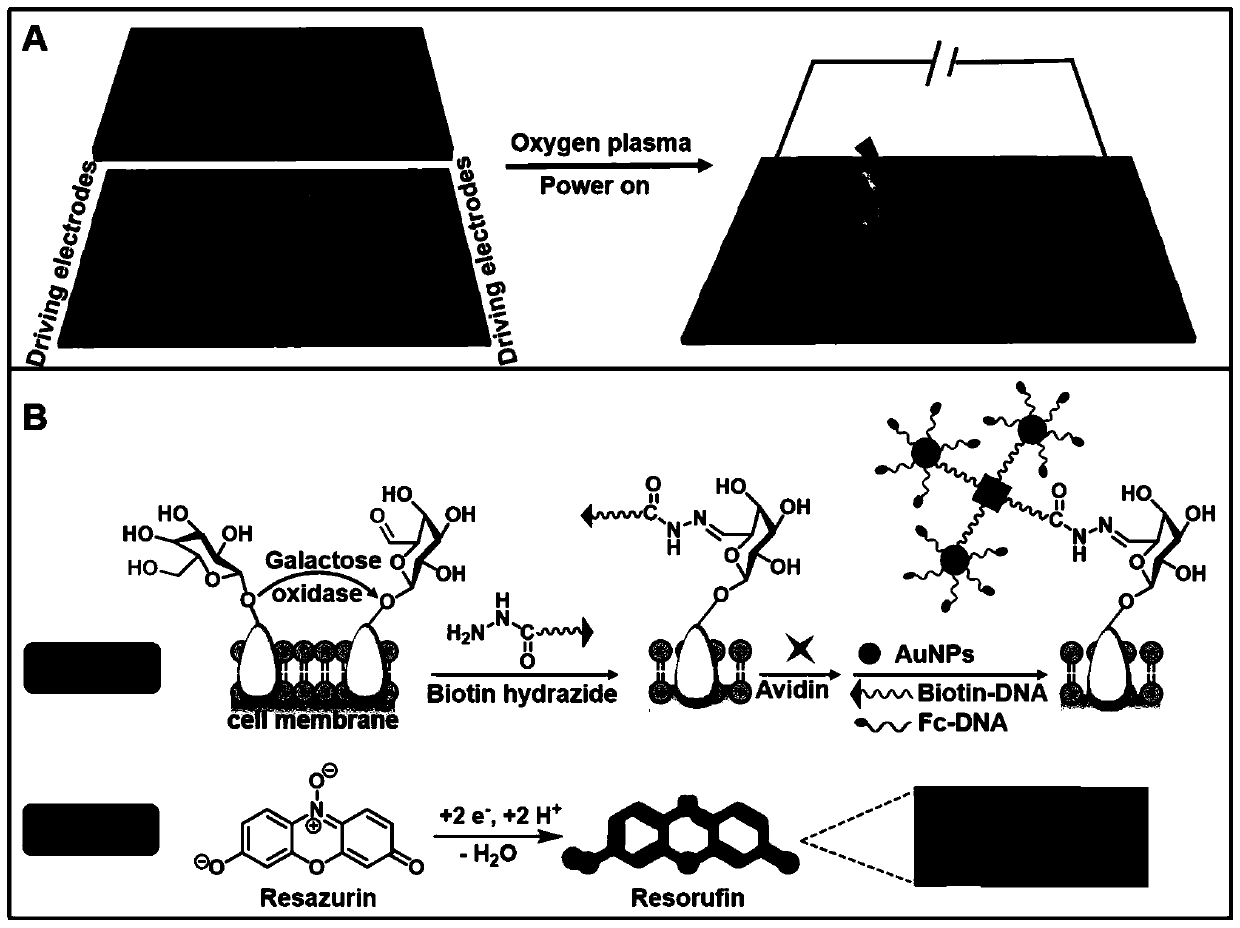
[0050] HepG2 cells were inoculated at a certain concentration in the anode microfluidic channel of the bipolar electrode and incubated for 6 hours. After the cells adhered to the wall, galactose oxidase was added to oxidize the hydroxyl on the galactose on the cell surface to aldehyde groups, and aniline catalyzed biotin The hydrazide reacts to form a hydrazone complex, thus introducing the biotin target on the cell surface, using avidin as a bridge, and binding to the Bio-DNA-AuNPs-Fc-DNA nanoprobe. Resazurin molecules are added to the cathode pool of the bipolar electrode. Under a certain external potential, the Fc introduced by the anode is oxidized, and the non-fluorescent resazurin at the cathode is reduced to highly fluorescent resorufin, which is quantified by the fluorescence intensity of the cathode. The expression level of galactose on the surface of anode cells was detected.
[0051] at 1.0 x 10 6 cells mL -1 , in the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Average hydrated particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com