Production method of semaglutide precursor
A technology of semaglutide and expression vector, which is applied in the field of genetic engineering and can solve the problems of low purity and low yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Construction of Recombinant Engineering Bacteria
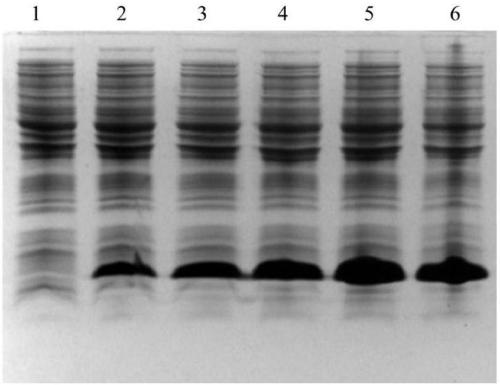
[0033] The semaglutide precursor gene expressed in tandem was integrated with the plasmid pET-27b(+) using the whole genome synthesis technology to obtain sequence cDNA, the restriction enzyme cutting site is NdeI / XhoI, and the induced expression electrophoresis is as follows figure 1 As shown, the recombinant plasmid was transformed into the host Escherichia coli (Escherichia coli) BL21 (DE3) by chemical transformation to construct the recombinant engineering bacteria. After sequencing, the sequence of the engineered bacteria was consistent with the designed sequence.
[0034] Enzymes and Reagents:
[0035] The restriction endonucleases used in the design of molecular biology operations in the examples can be obtained from TAKARA company, and the corresponding operation steps are completely carried out in accordance with the relevant product instructions.
[0036] The agarose gel recovery kit and plasmid extraction k...
Embodiment 2
[0044] High-density fermentation and induced expression
[0045] Inoculate the positive recombinant engineered bacteria selected in the above Example 1 into 10mL LB liquid medium according to the inoculation amount of 1%, and cultivate them at 30°C and 200rpm to OD600=12~15. The inoculum was inoculated in 100mL LB liquid medium, shaken at 200rpm at 30°C, and the OD 600 In the process of about 4, it was inserted into a 2.5L fermentation medium for high-density culture. The initial fermentation temperature is 34°C, the stirring speed is 200rpm, the ventilation rate is 2L / min, and the pH is 6.7, and then the stirring speed and ventilation rate are continuously increased to 800rpm and 8L / min to maintain the dissolved oxygen above 30%. Density fermentation requires a large amount of foreign air. If the supply of foreign air is insufficient, it will not only keep the respiration of the bacteria, limit the production and reproduction of the bacteria, but also accumulate some metabol...
Embodiment 3
[0052] Renaturation of recombinant tandem expressed semaglutide precursor
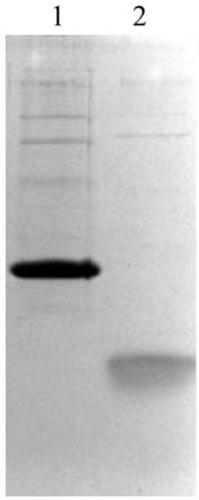
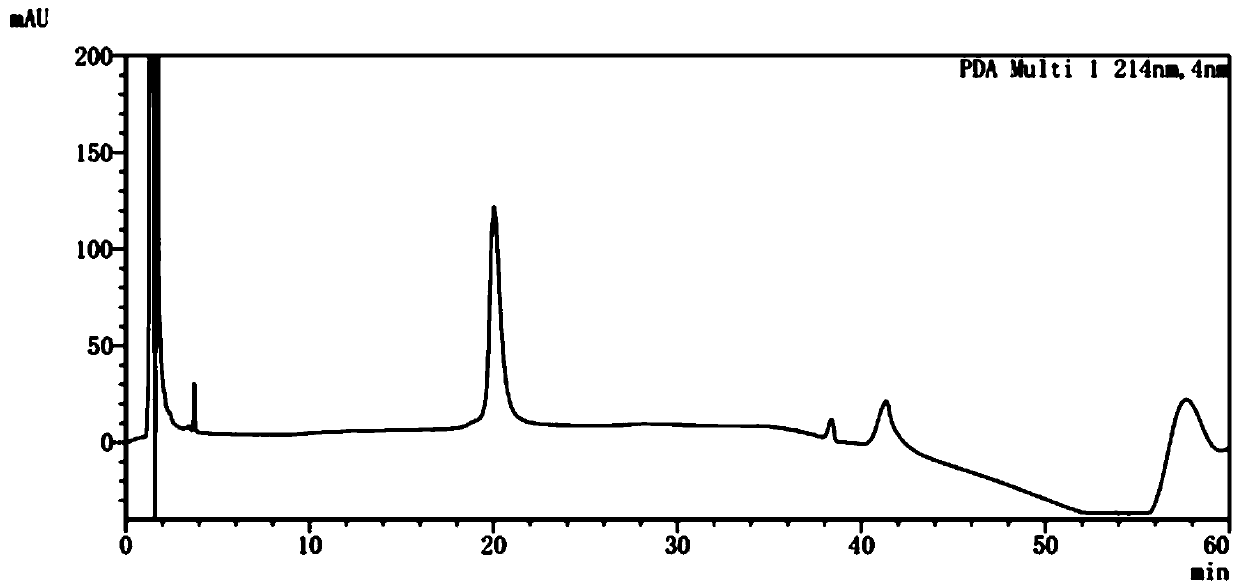
[0053] Centrifuge the engineered bacterium culture medium after the induced expression in Example 2, take the thalline, add the breaking buffer according to the ratio of 1:10 (w:v), and use the ATS homogenizer 850bar, break the bacteria twice at a frequency of 40Hz, 8500rpm Centrifuge for 30 min to collect inclusion bodies. The obtained semaglutide pre-inclusion bodies expressed in series were stirred evenly with 0.5M Tris-HCl pH 8.5 buffer solution, and then 0.5% SDS and 1% Triton X-100 were added to obtain a solution ratio of 4% (w:v) Stir the protein mixture solution with a magnetic stirrer at room temperature for 3-4 hours to slowly dissolve the precipitate; centrifuge at 10,000 rpm for 10 min at 4°C and discard the precipitate. The obtained supernatant was diluted 10 times with deionized water, and then filtered with a 0.22 μm microporous membrane to obtain the soluble protein.
[0054] Various ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com