Broad-spectrum antibacterial benzamide compound and preparation method and application thereof
A benzamide and compound technology, applied in the field of 3,4-dichloro-N-benzamide and its preparation, can solve the problem of improvement of antibacterial activity without improvement, Clostridium difficile, no improvement of antibacterial activity, etc. problem, to achieve the effect of simple structure, huge development potential and good killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of 3,4-dichloro-N-(5-nitrothiazol-2-yl)benzamide (Formula I)
[0032] One-step synthesis. The target compound is synthesized by the reaction of acid chloride and heterocyclic (or phenyl) amine compound. Weigh about 3 mmol of 2-amino-5-nitrothiazole (Formula III), put it into a 100 mL three-necked flask, add 15 mL THF (tetrahydrofuran), stir magnetically until it is completely dissolved, take an ice-salt bath, and cool to 0 After adding 1.2 to 1.5 eq (equivalent) triethylamine. 3.6 mmol of 3,4-dichlorobenzoyl chloride (formula II) was dissolved in 5 mL of THF, and then slowly added dropwise into the reaction system through a constant pressure dropping funnel. After the dropwise addition, the ice bath was removed, and an appropriate amount of DMAP (p-dimethylaminopyridine) was added after the reaction for half an hour. Use petroleum ether: ethyl acetate as a developer, monitor the reaction by thin-layer chromatography until there is no raw materi...
Embodiment 2
[0034] Example 2: Antibacterial application of 3,4-dichloro-N-(5-nitrothiazol-2-yl)benzamide (Formula I)
[0035] The minimum inhibitory concentration (MIC) value of the target derivative compound was determined by the microbroth dilution method recommended by the Clinical and Laboratory Standards Institute (CLSI) VET01-A4.
[0036] The bacteria used in the examples include quality control strains and clinical isolates. The quality control strains: Escherichia coli (ATCC25922) and Staphylococcus aureus (ATCC25923) are preserved by our laboratory. Bacillus cereus (CVCC2002), Pasteurella multocida (CVCC469), and Streptococcus equine (CVCC556) were purchased from the strain preservation bank of China Veterinary Drug Control Institute. Clinically isolated strains: Escherichia coli, Staphylococcus aureus, Salmonella, Klebsiella pneumoniae, Aeromonas, Enterococcus and other 35 strains of bacteria were isolated, identified and preserved by our laboratory.
[0037] The target product...
Embodiment 3
[0044] Example 3: Inhibitory effect of 3,4-dichloro-N-(5-nitrothiazol-2-yl)benzamide (formula I) on bacterial growth and reproduction
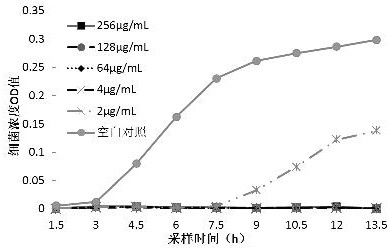
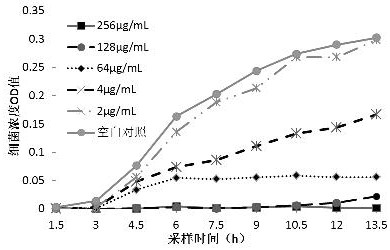
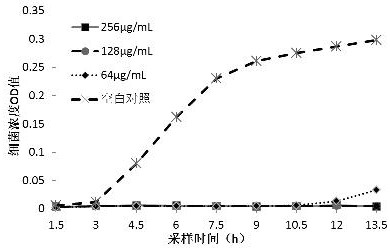
[0045] Bacillus cereus (CVCC2002), Staphylococcus aureus ( S. aureus 3) For the test strain, take 10 5 The concentration of cfu / mL bacterial solution is the initial experimental bacterial concentration. After adding different concentrations of 3,4-dichloro-N-(5-nitrothiazol-2-yl)benzamide (Formula I) and NTZ, at 37 After culturing in a shaker at 180 rpm at 180 °C, 1 mL was taken every 1.5 h to measure the OD values of different concentrations of bacterial solutions at 630 nm, and three replicates were set up to calculate the average value of OD. The antibacterial growth curve was drawn with time as the abscissa and OD value as the ordinate.
[0046] from Figure 1-4 As can be seen from the results in: before 7.5h, 2 µg / mL of 3,4-dichloro-N-(5-nitrothiazol-2-yl)benzamide (formula I) has always been effective against Bacillus cereus Very...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com