5-aminolevulinic acid synthetase mutant, and host cell and application thereof
A technology of aminolevulinic acid and host cells, which is applied to mutants of 5-aminolevulinic acid synthase and its host cells and application fields, and can solve the problems of few and low expression activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Example 1. Structural Analysis of ALA Synthetase
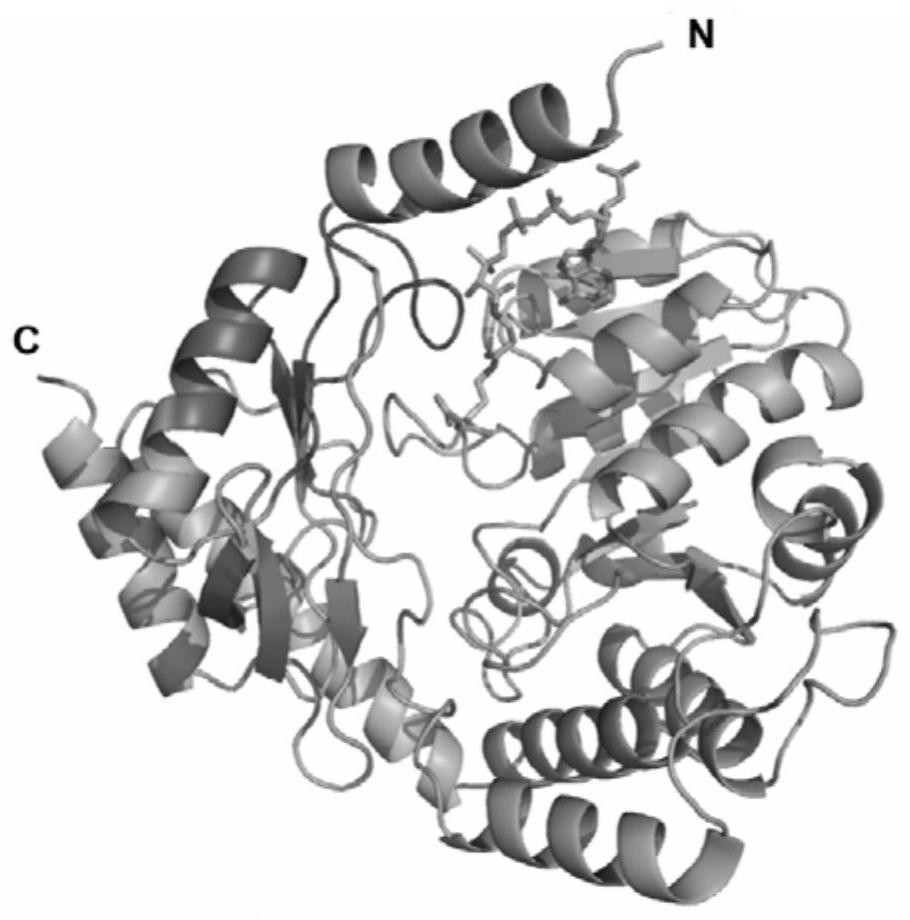
[0150] The literature reported the protein structure of ALA synthase (RcA) derived from Rhodobacter capsulata (PDB database number: 2BWN) and its substrates glycine (PDB database number: 2BWP) and succinyl-CoA (PDB Database number: 2BWO) co-crystallized protein structure, in which Arg374 site directly interacts with glycine, and Thr365 site directly interacts with succinyl-CoA (Astner et al. EMBO Journal 2005; 24:3166-3177). After structural analysis of the enzyme, it was found that the two regions of 332-348 and 358-374 in its amino acid sequence (shown in purple) are related to substrate binding (such as figure 1 shown), the above region includes the Arg374 site that directly interacts with glycine and the Thr365 site that directly interacts with succinyl-CoA, and its flexible C-terminus is relatively close to the spatial position of the above two regions. The inventors speculate that the mutation of the flexible C-t...
Embodiment 2
[0152] Example 2: Sequence Analysis of ALA Synthetase from Different Species
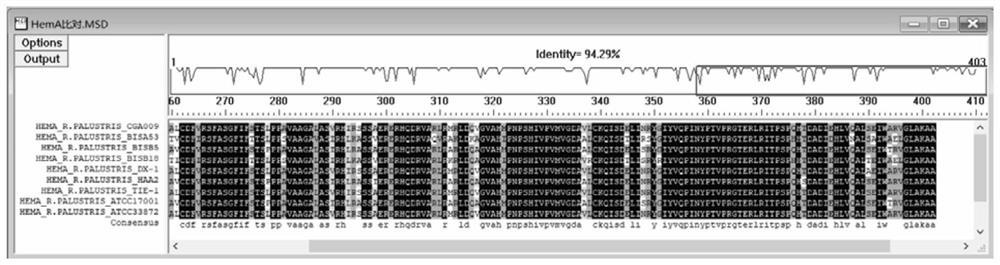
[0153] First, the inventors compared and analyzed the amino acid sequences of HemA of different Rhodopseudomonas palustris subspecies that have been reported, and found that the sequence identity between different strains was very high, reaching 94%, and the 403rd position of the C-terminal Amino acid residues are highly conserved, all of which are alanine (such as figure 2 shown).
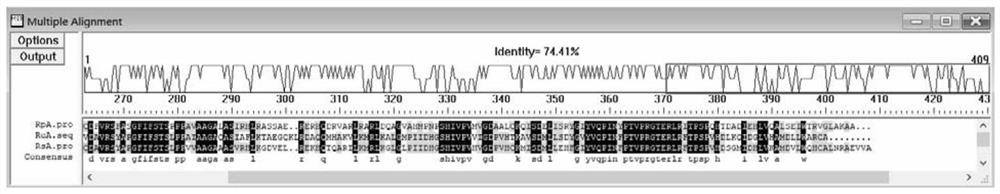
[0154] The present inventors further expanded the scope of sequence alignment, and performed Blast alignment at NCBI with the amino acid sequence of Rhodopseudomonas palustris ATCC17001 HemA (RpA), and analyzed the sequence annotated as ALA synthetase. Although the sources of reported ALA synthetases are different, most of the amino acid sequences of ALA synthetase have alanine at the C-terminal end or the 403rd position. The specific comparison results are as image 3 shown.
[0155] For example, the ALA synthetas...
Embodiment 3
[0156] Embodiment 3. Construction of Rhodopseudomonas palustris ATCC17001HemA (RpA) mutant vector
[0157] Utilize the Stratagene Series XL-II site-directed mutagenesis kit, designed 39 pairs of primers (see Table 2), using the pEC-XK99E-hemA wild-type plasmid as a template (the construction process refers to the construction and fermentation of the pathway for the synthesis of 5-aminolevulinic acid by Corynebacterium glutamicum Optimization [J]. Biotechnology Bulletin, 2017,33(01):148-156.), using the above primers for PCR amplification, respectively mutating the 403rd amino acid residue of Rhodopseudomonas palustris ATCC17001HemA(RpA) Arginine (R), asparagine (N), aspartic acid (D), cysteine (C), glutamine (Q), glutamic acid (E), glycine (G), Histidine (H), Isoleucine (I), Leucine (L), Lysine (K), Methionine (M), Phenylalanine (F), Proline (P ), Serine (S), Threonine (T), Tryptophan (W), Tyrosine (Y), Valine (V). The PCR reaction conditions are: 95°C for 5min, 10 cycle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com