Troger's base conjugated microporous polymer photocatalyst, and preparation method and application thereof
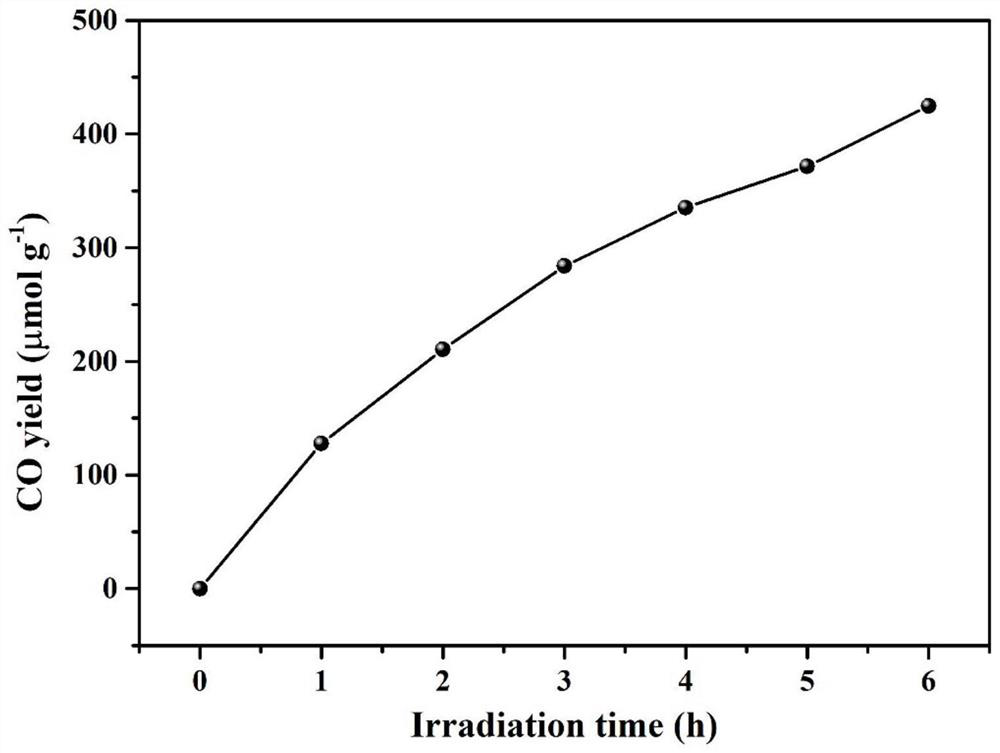
A technology towards Geer bases and microporous polymers, applied in the direction of organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problems of insufficient absorption and utilization of visible light , to achieve excellent CO2 adsorption capacity, large application potential, and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The reaction equation of the present embodiment is as follows:
[0035]
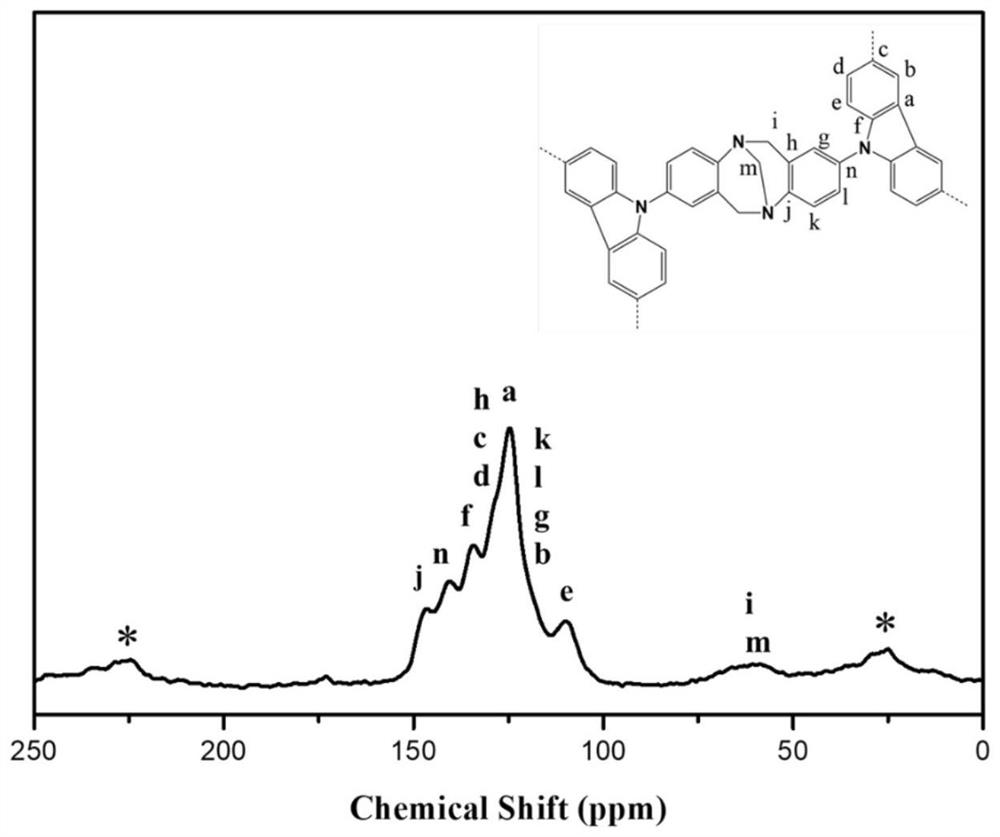
[0036] Dissolve 2.369g diiodochauger base (5.0mmol) in 40mL anhydrous N,N-dimethylformamide, add 3.344g carbazole (20mmol), 2.764g potassium carbonate (20mmol), 0.095g iodine Cuprous chloride (0.5mmol) and 0.009g 1,10-phenanthroline (0.05mmol) were reacted at 150°C for 24h under nitrogen protection. Cool to room temperature, remove the solvent, and purify the product by column chromatography (ethyl acetate / petroleum ether=1:3) to obtain a light brown carbazole-based conjugated microporous polymer monomer.
[0037]Disperse 0.203g (0.367mmol) of carbazole-based conjugated microporous polymer monomer in 25mL of anhydrous chloroform, then add 0.595g (3.67mmol) of ferric chloride, and react at 60°C for 24h under nitrogen protection. Then 50 mL of methanol was added to the reaction mixture, and the resulting mixture was stirred for another 1 hour, and the precipitate was collected by filtration and ...
Embodiment 2
[0043] The reaction equation of the present embodiment is as follows:
[0044]
[0045] With 20ml of N,N-dimethylformamide and 20ml of triethylamine mixed solution as the reaction medium, add 0.356g of diiodo Chaoger base (0.75mmol), 0.075g of 1,3,5-triethynylbenzene ( 0.5mmol), 0.029g Pd (pph 3 ) 4 (0.025mmol) and 0.005g CuI (0.025mmol), reacted at 80°C for 24h under nitrogen protection. Collect the precipitate after the reaction, stir the precipitate in hydrochloric acid solution for 1 hour, wash with water, stir in a mixed solution of acetone, toluene and methanol for 1 hour, dry, and perform Soxhlet extraction with a mixed solution of methanol and tetrahydrofuran to obtain a yellow Conjugated microporous polymer in powder form.
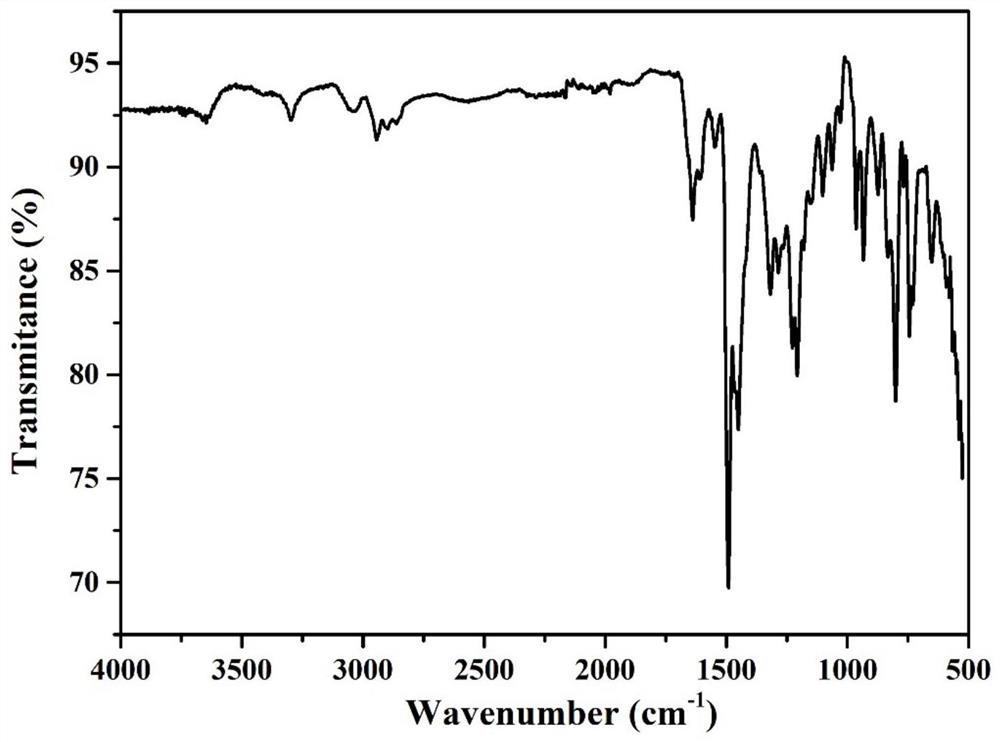
[0046] Figure 5 It is the infrared spectrogram of the conjugated microporous polymer prepared in Example 2. Among them at 1316cm -1 and 1097cm -1 It is the stretching vibration absorption peak of C-N outside the ring, which is the carbo...
Embodiment 3
[0050] The reaction equation of the present embodiment is as follows:
[0051]
[0052] With 20ml of N,N-dimethylformamide and 20ml of triethylamine mixed solution as the reaction medium, add 0.356g of diiodochauger base (0.75mmol), 0.063g of 1,4-diethynylbenzene (0.5mmol ), 0.029g Pd (pph 3 ) 4 (0.025mmol) and 0.005g CuI (0.025mmol), reacted at 80°C for 24h under nitrogen protection. Collect the precipitate after the reaction, stir the precipitate in hydrochloric acid solution for 1 hour, wash with water, stir in a mixed solution of acetone, toluene and methanol for 1 hour, dry, and perform Soxhlet extraction with a mixed solution of methanol and tetrahydrofuran to obtain a yellow Conjugated microporous polymer in powder form.
[0053] Figure 8 It is the infrared spectrogram of the conjugated microporous polymer prepared in Example 3. Among them at 1316cm -1 and 1097cm -1 It is the stretching vibration absorption peak of C-N outside the ring, which is the carbon-ni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com