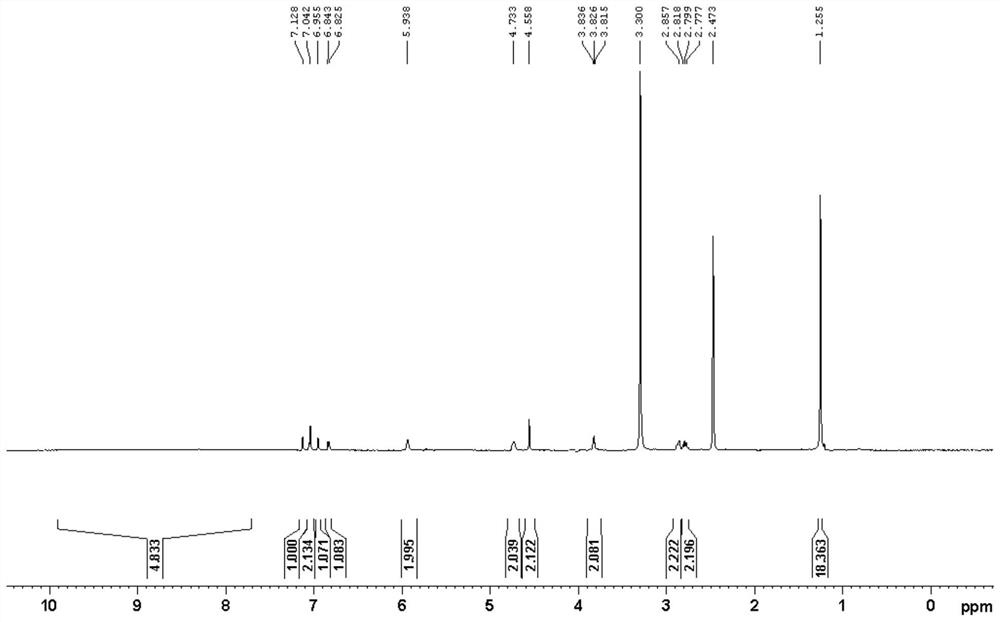
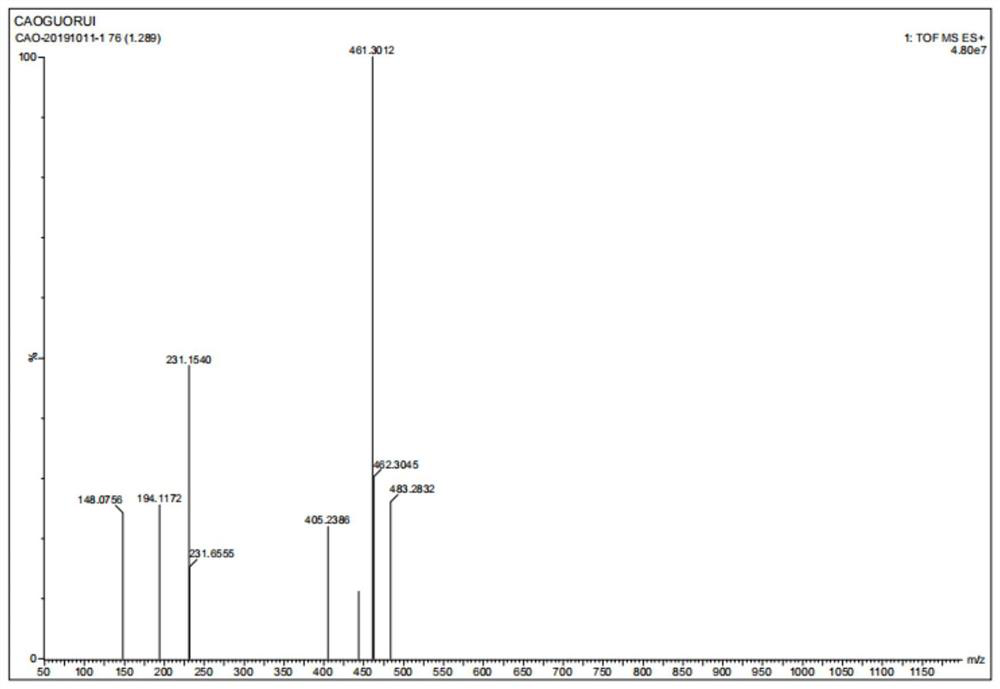
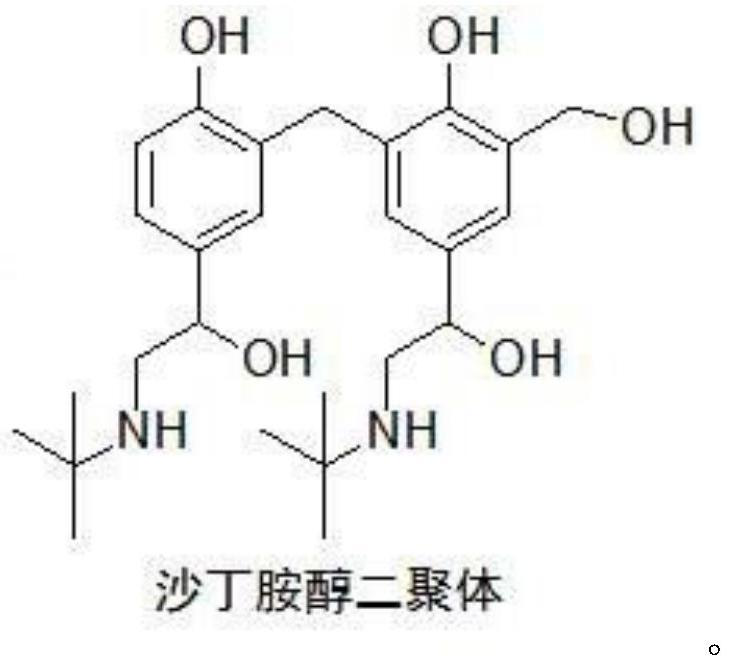
Preparation method of salbutamol dimer
A technology for salbutamol dimer and tert-butylamine, which is applied in the field of preparation of albuterol dimer, and achieves the effects of increasing yield, concise synthesis steps and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 1) Put p-bromophenol (3.46g, 20mol) and 38% first aqueous formaldehyde solution (3.16g, 40mmol) in a 50mL three-necked flask, heat to reflux, and stir for 6 hours. Turn off the heating, cool naturally to room temperature, add 10% sodium hydroxide aqueous solution (8mL) and 38% second formaldehyde aqueous solution (1.97g, 25mmol) to it successively, stir at room temperature for 12 hours, stop the reaction, and adjust the pH with 1mol / L hydrochloric acid to 8-9, extracted with ethyl acetate (10 mL), dried over anhydrous sodium sulfate, suction filtered, the filtrate was concentrated under reduced pressure, and the residue was subjected to silica gel column chromatography (200-300 mesh column chromatography silica gel, eluent 10- 20% ethyl acetate / petroleum ether) to obtain intermediate I 6.05g, yield 78%, white solid.
[0051] The reaction equation is:
[0052]
[0053]2) Put Intermediate I (3.88g, 10mmol) in a 50mL single-necked bottle, add N,N-dimethylformamide (DMF...
Embodiment 2
[0071] 1) Put p-bromophenol (3.46g, 20mol) and 38% first aqueous formaldehyde solution (2.37g, 30mmol) in a 50mL three-necked flask, heat to reflux, and stir for 6 hours. Turn off the heating, cool to room temperature naturally, add 10% sodium hydroxide aqueous solution (8mL) and 38% second formaldehyde aqueous solution (1.58g, 20mmol) to it successively, stir at room temperature for 12 hours, stop the reaction, and adjust the pH with 1mol / L hydrochloric acid to 8-9, extracted with ethyl acetate (10 mL), dried over anhydrous sodium sulfate, suction filtered, the filtrate was concentrated under reduced pressure, and the residue was subjected to silica gel column chromatography (200-300 mesh column chromatography silica gel, eluent 10- 20% ethyl acetate / petroleum ether) to obtain intermediate I 5.78g, yield 74.5%, white solid.
[0072] The reaction equation is:
[0073]
[0074] 2) Put Intermediate I (3.88g, 10mmol) in a 50mL single-necked bottle, add N,N-dimethylformamide (...
Embodiment 3
[0090] 1) Put p-bromophenol (3.46g, 20mol) and 38% first aqueous formaldehyde solution (4.75g, 60mmol) in a 50mL three-neck flask, heat to reflux, and stir for 6 hours. Turn off the heating, cool to room temperature naturally, add 10% sodium hydroxide aqueous solution (8mL) and 38% second formaldehyde aqueous solution (2.37g, 30mmol) to it successively, stir at room temperature for 12 hours, stop the reaction, and adjust the pH with 1mol / L hydrochloric acid to 8-9, extracted with ethyl acetate (10 mL), dried over anhydrous sodium sulfate, suction filtered, the filtrate was concentrated under reduced pressure, and the residue was subjected to silica gel column chromatography (200-300 mesh column chromatography silica gel, eluent 10- 20% ethyl acetate / petroleum ether) to obtain intermediate I 5.89g, yield 75.9%, white solid.
[0091] The reaction equation is:
[0092]
[0093]2) Put Intermediate I (3.88g, 10mmol) in a 50mL single-necked bottle, add N,N-dimethylformamide (DMF...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com