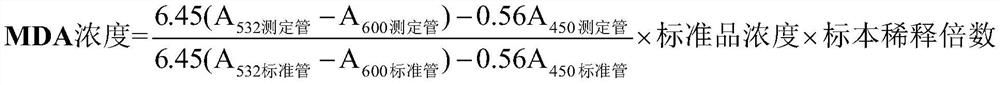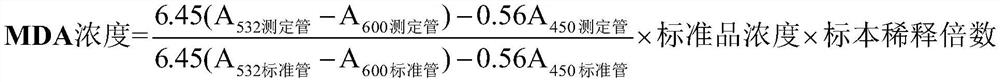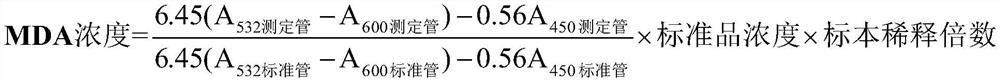Quantitative determination method and kit for malondialdehyde content in seminal plasma
A technology for quantitative determination of malondialdehyde, applied in the field of in vitro detection of sperm biochemical indicators, can solve the problems of large error in test results, cumbersome operation, high test results, etc., and achieve the effect of increasing absorbance value, increasing sensitivity and eliminating influence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Reagent composition: 10umol / L malondialdehyde solution (standard product), 2mol / L trichloroacetic acid (specimen treatment solution), TBA reagent.
[0028] Reagent preparation:
[0029] Preparation of 0.02mol / L hydrochloric acid: Measure 10mL of 0.24mol / L hydrochloric acid and dilute with water to 120mL.
[0030] Preparation of malondialdehyde standard (10umol / L) (molecular weight: 164.2): Accurately weigh 16.42mg of malondialdehyde dimethyl acetal (TMP), add 0.02mol / L hydrochloric acid 1000ml, stir at room temperature for 60min, after TMP is fully dissolved , aliquoted into 0.5ml / bottle, stored at 2-8°C.
[0031] Preparation of specimen treatment solution: Accurately weigh 16.34g of trichloroacetic acid, dissolve in 50ml of deionized water, and store at 2-8°C.
[0032] Preparation of 1mol / L sodium hydroxide solution: Accurately weigh 4g of sodium hydroxide, dissolve in 100ml of deionized water, and store at 2-8°C.
Embodiment 2
[0074] A quantitative detection kit for malondialdehyde content in seminal plasma, comprising, malondialdehyde standard product, specimen treatment solution and TBA reagent; wherein, the concentration of the malondialdehyde standard product is 10umol / L, and the specimen treatment solution is 2mol / L trichloroacetic acid, the TBA reagent includes 0.8% thiobarbituric acid solution and 0.1mg / L ferric sulfate solution. The kit includes a centrifuge tube with a specification of 0.5-5.0ml, and the malondialdehyde standard, specimen processing solution and TBA reagent are separately packed in bottles.
[0075] In the above example, the preparation steps of malondialdehyde standard (10umol / L): Accurately weigh 16.42mg of malondialdehyde dimethyl acetal (TMP), add 1000ml of 0.02mol / L hydrochloric acid, fully stir at room temperature for 60min, and wait until TMP is fully dissolved Finally, aliquot into 0.5ml / bottle and store at 2-8°C.
[0076] Preparation steps of specimen treatment so...
Embodiment 3
[0080] A quantitative detection kit for malondialdehyde content in seminal plasma, comprising, malondialdehyde standard product, specimen treatment solution and TBA reagent; wherein, the concentration of the malondialdehyde standard product is 1 μmol / L, and the specimen treatment solution is 0.5mol / L trichloroacetic acid, the TBA reagent includes 0.1% thiobarbituric acid solution and 0.05mg / L ferric sulfate solution. The kit includes a centrifuge tube with a specification of 0.5-5.0ml, and the malondialdehyde standard, specimen processing solution and TBA reagent are separately packed in bottles.
[0081] In the above example, the preparation steps of malondialdehyde standard product (1umol / L): Accurately weigh 1.64mg of malondialdehyde dimethyl acetal (TMP), add 1000ml of 0.01mol / L hydrochloric acid, stir well at room temperature for 10min, and wait until TMP is fully dissolved Afterwards, aliquot into 0.5ml / bottle and store at 8°C.
[0082] Preparation steps of specimen tre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com