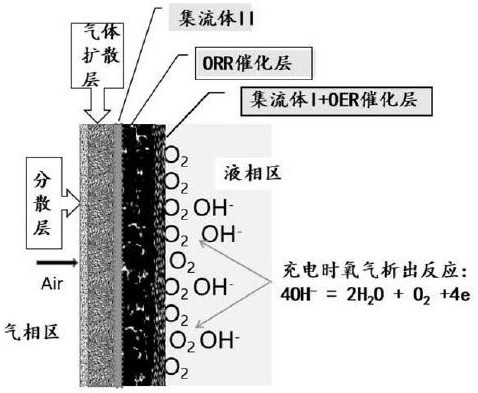
A multilayer functional structure and durable and stable electrically chargeable air electrode and its manufacturing method
A technology of air electrodes and functional structures, applied in the direction of battery electrodes, structural parts, electrical components, etc., to achieve the effects of stable long-term charge and discharge cycles, stable structure, and shortened air diffusion distance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The preparation process of the above-mentioned electrically chargeable air electrode can be as follows:
[0061] To prepare the OER composite catalytic layer, sintering method or paste method can be used:
[0062] 1.1. Sintering method
[0063] (1) Weigh non-noble metal transition metal salt compounds, oxides or hydroxides in a reagent flask, add distilled water or other solvents to form a solution, and control the total concentration of non-noble metal transition metal ions to 0.5-3.0M, preferably 1.0 -2.0M.
[0064] (2) After the current collector I is cleaned and degreased by ultrasonic waves in acetone solution, it is impregnated with the above solution, and then sintered at 200-400°C, the preferred temperature is 250-300°C, the sintering time is 30-180 minutes, and the preferred time is 60-120 Minutes, repeat the sintering process 1-2 times to form an apparently uniform OER composite catalytic layer.
[0065] 1.2. Ointment method
[0066] 1) Weigh non-noble metal...
Embodiment 1
[0085] OER catalyst and paste method to prepare OER composite catalytic layer:
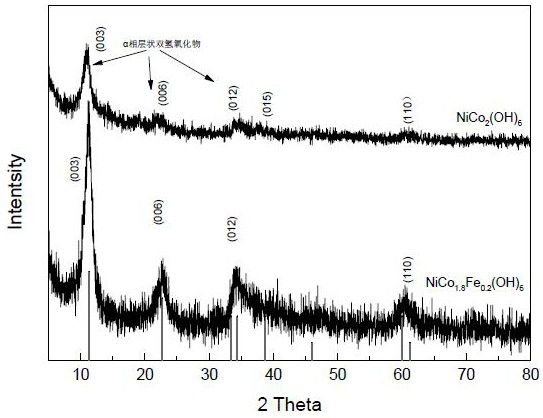
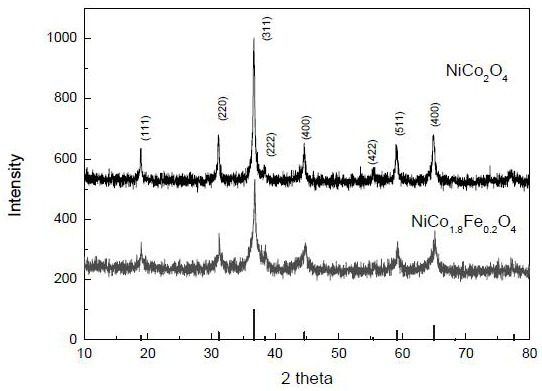
[0086] Weigh nickel nitrate, cobalt nitrate (or ferric nitrate) dissolved in 25 ml of absolute ethanol to form a solution and magnetically stir to control the total metal ion concentration to 0.6-1.0M, wherein: the concentration ratio of nickel to cobalt ions is 2.5:5.0 , or the concentration ratio of nickel, cobalt and iron ion is 2.5:4.5:0.5, add 0.6N 60 milliliters of ammonia water dropwise to the range of pH value 8-9 in the mixed solution, and continue magnetic stirring to mix evenly, coprecipitate is centrifuged, Filter and wash with distilled water to remove other ions, and dry at 80°C to obtain a multi-component transition metal hydroxide composite catalyst. X-ray results see image 3 , structural analysis shows that the binary Ni-Co and ternary Ni-Co-Fe hydroxide catalysts have a hydrotalcite-like layered hydroxide structure, which is composed of (003), (006), (012) characteristic peaks ...
Embodiment 2
[0088] OER catalyst and sintering method to prepare OER composite catalytic layer:
[0089] Take metal foam nickel with a porous three-dimensional structure with a size of 3.0*5.5*0.15cm as the current collector, ultrasonically clean it in acetone solution for 30 minutes, corrode with dilute hydrochloric acid for 1 minute and rinse with distilled water for 3 times, then impregnate it in a metal containing nickel and cobalt. ion solution, wherein the concentration ratio of nickel and cobalt ions is 1:(2-X)(0≤X≤2), or immersed in an ethanol mixed solution containing nickel, cobalt and iron ions, wherein nickel, cobalt and iron The ion concentration ratio is 1:(2-X):X (0.1≤X≤2); the concentration of total metal ions in the solution is 2.0M; after the nickel foam is impregnated in the above mixed ion solution, it is sintered at 300°C for 40 minutes. Repeated impregnation and sintering for 2 times to obtain OER composite catalytic layer, in which the catalyst loading is 8-12mg / cm ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com