Nitrogen-doped graphene/cobalt-zinc ferrite composite aerogel wave-absorbing material and preparation method thereof
A composite airgel and wave-absorbing material technology, which is applied in the field of nitrogen-doped graphene/cobalt-zinc ferrite composite airgel wave-absorbing material and its preparation, can solve the problems of high density, mismatched ferrite impedance, Solve problems such as weak electromagnetic wave absorption ability, achieve the effect of wide frequency band, excellent wave absorption performance, and low filling ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
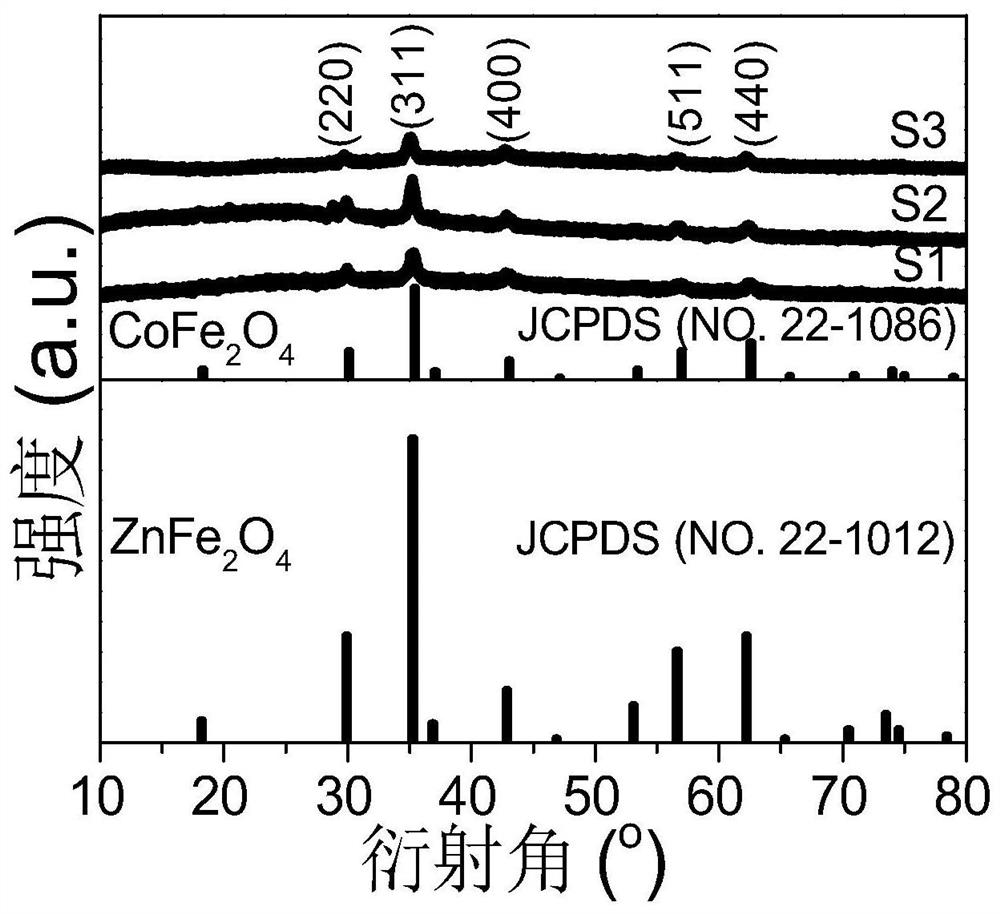
[0057] (1) Take a 200mL beaker, add 60mL ethylene glycol, and then add 1.08g (4mmol) ferric chloride hexahydrate (FeCl 3 ·6H 2 O), 0.24g (1mmol) cobalt chloride hexahydrate (CoCl 2 ·6H 2 O) and 0.14g (1mmol) zinc chloride (ZnCl 2 ), stirring vigorously to dissolve it completely, among them, zinc ions (Zn 2+ ), cobalt ions (Co 2+ ) and iron ions (Fe 3+ ) in a molar ratio of 1:1:4, then add a certain amount of 5.4g sodium acetate, stir for 15 minutes until completely dissolved, and add sodium acetate as an electrostatic stabilizer;
[0058] (2) Add quantitatively 1.5g polyethylene glycol (PEG), stir at 50°C for 2h to dissolve to obtain the first mixed dispersion; PEG as a polymer stabilizer must be stirred for 2h at 50°C in a water bath to dissolve, otherwise it will be difficult Dissolve to form a uniform dispersion;
[0059] (3) Transfer the first mixed dispersion to a reaction kettle with a volume of 100mL, and conduct a solvothermal reaction at 200°C for 8 hours to ob...
Embodiment 2
[0071] (1) Take a 200mL beaker, add 60mL ethylene glycol, and then add 1.08g (4mmol) FeCl3 ·6H 2 O, 0.24g (1mmol) CoCl 2 ·6H 2 O and 0.14g (1mmol) ZnCl 2 , stirred vigorously to dissolve completely, among them, Zn 2+ 、Co 2+ and Fe 3+ The molar ratio is 1:1:4, then add a certain amount of 5.4g sodium acetate, stir for 15min until completely dissolved;
[0072] (2) Add a certain amount of 1.5g PEG, stir at 50°C for 2 hours to dissolve it to obtain the first mixed dispersion;
[0073] (3) Transfer the first mixed dispersion liquid to a reaction kettle with a volume of 100mL, and conduct a solvothermal reaction at 200°C for 8 hours to obtain a product containing cobalt-zinc ferrite;
[0074] (4) After the reaction is finished, cool to room temperature, magnetically separate the product to obtain hydrous cobalt-zinc ferrite, wash with deionized water several times to make the pH of the hydrous cobalt-zinc ferrite reach neutral;
[0075] (5) After pre-freezing the hydrated co...
Embodiment 3
[0087] (1) Take a 200mL beaker, add 60mL ethylene glycol, and then add 1.08g (4mmol) FeCl 3 ·6H 2 O, 0.24g (1mmol) CoCl 2 ·6H 2 O and 0.14g (1mmol) ZnCl 2 , stirred vigorously to dissolve completely, among them, Zn 2+ 、Co 2+ and Fe 3+ The molar ratio is 1:1:4, then add a certain amount of 5.4g sodium acetate, stir for 15min until completely dissolved;
[0088] (2) Add a certain amount of 1.5g PEG, stir at 50°C for 2 hours to dissolve it to obtain the first mixed dispersion;
[0089] (3) Transfer the first mixed dispersion liquid to a reaction kettle with a volume of 100mL, and conduct a solvothermal reaction at 200°C for 8 hours to obtain a product containing cobalt-zinc ferrite;
[0090] (4) After the reaction is finished, cool to room temperature, magnetically separate the product to obtain hydrous cobalt-zinc ferrite, wash with deionized water several times to make the pH of the hydrous cobalt-zinc ferrite reach neutral;
[0091] (5) After pre-freezing the hydrated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
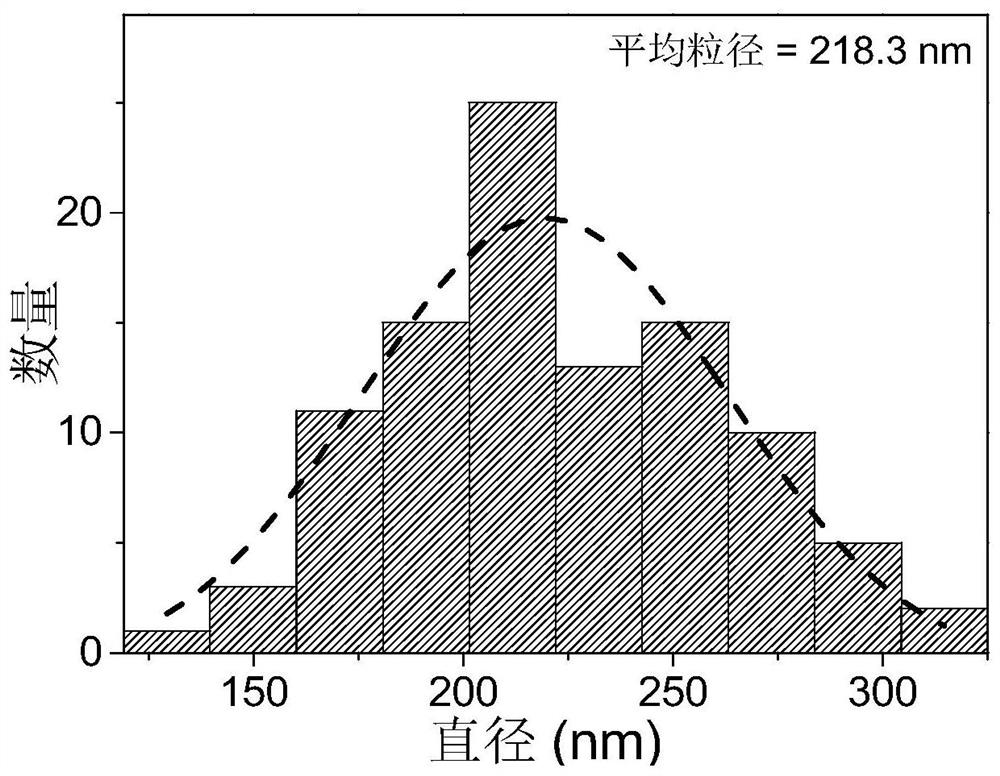
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com