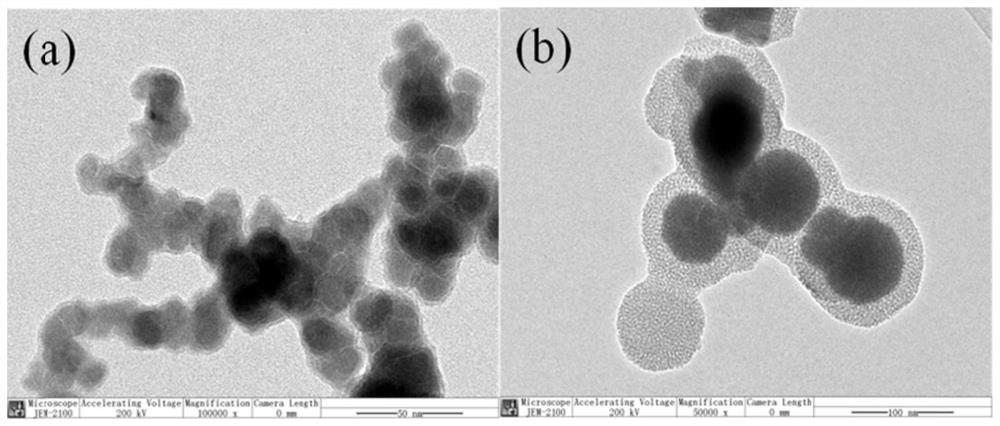
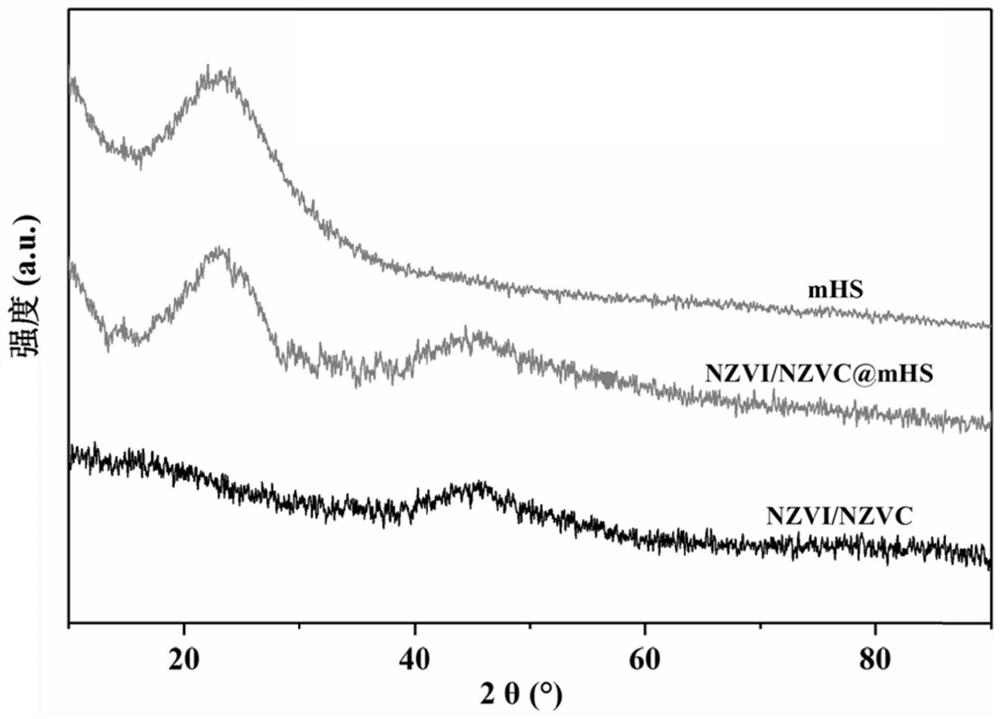
Hydrated mesoporous silica coated nano iron-cobalt bimetallic composite material and application thereof
A silica, hydrated mesoporous technology, used in metal/metal oxide/metal hydroxide catalysts, oxidized water/sewage treatment, water pollutants, etc. High requirements for electrodes, etc., to achieve the effects of improving dispersibility and reactivity, increasing exposed active sites, and overcoming easy agglomeration and inactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] This embodiment provides a method for preparing a hydrated mesoporous silica-coated nano-iron-cobalt bimetallic composite, which specifically includes the following steps:
[0020] Step 1, 0.496g FeSO 4 ·7H 2 O and 0.502g CoSO 4 ·7H 2 O is dissolved in the mixed solution of 95mL water and ethanol, wherein the volume ratio of water and ethanol is (45-50): (50-45), stir to make it dissolve completely;
[0021] Step 2. Weigh 0.05g-0.06gNaBH 4 Dissolve in 50ml of deionized water and place in a three-necked flask. Will hold NaBH 4 The three-necked flask of solution was placed into the sonicator (50-70% power indication). Under the protection of nitrogen, add the mixed solution of step 1 dropwise to the solution at a rate of 3 mL / min, after the dropwise addition is completed. Continue to stir for 30-60 minutes, the solution turns black or dark brown, and obtains nano-iron-cobalt bimetallic suspension;
[0022] Step 3, continue to add 5mL NH to the three-necked flask ...
Embodiment example 2
[0033] This embodiment provides the method for removing tetrabromobisphenol A in groundwater by the iron-cobalt bimetallic composite coated with hydrated mesoporous silica obtained in embodiment 1, which specifically includes the following steps:
[0034] Step 1. Take five 100mL serum bottles and add 50ml tetrabromobisphenol A (10mg / L) solution respectively. Take out 4 serum bottles again and add the hydrogen peroxide of 20mmol / L, only stay the serum bottle that only contains 50ml tetrabromobisphenol A (10mg / L). The solutions in all 5 serum bottles were adjusted to pH=7.
[0035] Step 2. Weigh 1 g / mL of the iron-cobalt bimetallic composite coated with hydrated mesoporous silica obtained in Example 1 (containing 0.25 g / mL nano-iron-cobalt bimetal), 0.25 g / mL nano-iron-cobalt Bimetallic and 0.75 g / mL hydrated mesoporous silica, were added separately to serum bottles containing hydrogen peroxide. A serum bottle containing only tetrabromobisphenol A was used as a control. In ad...
Embodiment example 3
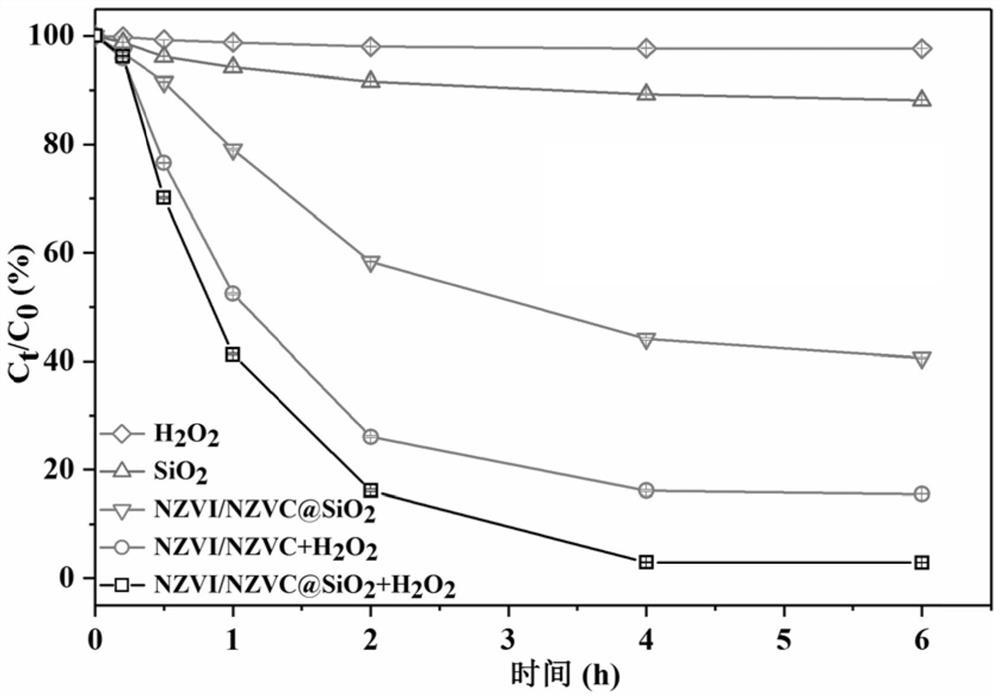
[0042] This example provides the iron-cobalt bimetallic composite material coated with hydrated mesoporous silica obtained in Example 1, and the cyclic degradation method. Specifically include the following steps:
[0043] Step 1. Carry out magnetic separation on the iron-cobalt bimetallic composite coated with hydrated mesoporous silica after the reaction in Example 2, wash with deionized water and absolute ethanol in turn, and obtain the composite after the reaction after repeated washing three times. Material;
[0044] Step 2. Add the composite material to a serum bottle containing 50ml TBBPA (10mg / L) and 20mmol / L hydrogen peroxide, and adjust the pH=7.
[0045] Step 3. Put the serum bottle in a constant temperature shaking incubator. During the reaction, at specified time intervals (0, 0.2, 0.5, 1, 2, 4, and 6 h), collect 1 mL of water samples (filtered with a 0.22 μm organic filter) and immediately quench the reaction with 0.5 μmL of tert-butanol .
[0046] Step 4, me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com