Alpha, omega-type chain transfer agent for reversible addition-fragmentation chain transfer RAFT polymerization and preparation method and application thereof
A technology of fragmentation chain transfer and chain transfer agent, which is applied in the field of α, and can solve the problems of many steps and poor stability of trithioesters, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
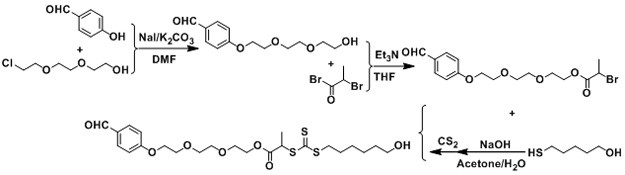
[0050]The preparation method of α, ω-type chain transfer agent for reversible addition-breaking chain transfer RAFT polymerization, including the following steps:
[0051](1) With 2- (2- (2-chloroethoxy) ethoxy) ethanol, hydroxybenzaldehyde is used in the use of raw materials, 2- (2- (2- (2-) under the action of potassium carbonate and sodium iodide. (4-formylphenoxy) ethoxy) ethoxy) ethanol;
[0052](2) 2- (2- (2- (4-formylphenoxy) ethoxy) ethoxy) ethanol reacts with 2-bromopropyl bromide under triethylamine, 2- (2- (2- (4-formylphenoxy) ethoxy) ethoxy) ethyl 2-bromopropane;
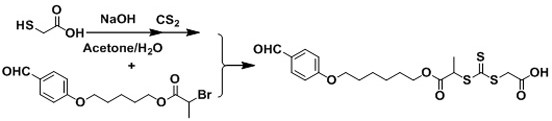
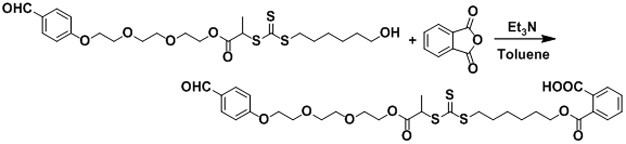
[0053](3) 2- (2- (2- (4-formylphenoxy) ethoxy) ethoxy) ethyl 2-bromopropane with mercaptohydrol, mercaptoacetic acid, mercaptoacid, And the product reacted in the sulfide solution is reacted in the sodium hydroxide solution, and finally obtains a chain transfer agent containing an aldehyde group and the end containing a hydroxyl group or a carboxyl group.
[0054]Next, the present invention will be further described with...
Embodiment 1
[0055]Example 1, a RAFT reagent for having one end is a hydroxyl structure of one end of an aldehyde group:
[0056]according tofigure 1 The synthetic roadmap is prepared to have an aldehyde group and the end is the specific steps of the RAFT reagent of the hydroxyl group as follows:
[0057]1) 2 - (2 - (2-chloroethoxy) ethoxy) ethanol (0.2 mol), hydroxybenzaldehyde (0.2 mol), potassium carbonate (0.2 mol) (0.2 mol) dissolved in 200 ml DMF (dimethylformamide) was reacted at 100 ° C for 24 hours. After cooling to room temperature, deionized water was added and extracted with ethyl acetate. The organic phase was collected. After drying over magnesium sulfate, the evaporation was evaporated to remove ethyl acetate, followed by separation by silica gel column chromatography (ethyl acetate / petroleum ether, volume ratio: 1 / 4), a light yellow liquid 30.6 g, yield 60% .
[0058]2) 2- (2- (2- (4-formylphenoxy) ethoxy) ethoxy) ethanol (0.12 mol) and triethylamine (0.12 mol) were dissolved in 100 ml ...
Embodiment 2
[0061]Example 2, a RAFT reagent for having one end is a fatty acid structure: one end is a fatty acid structure:
[0062]according tofigure 2 The synthetic road map is prepared by the RAFT reagent containing the aldehyde end of the carboxyl structure. The specific steps are as follows: Steps (1) and (2) in Example 1, step (3), mercaptoacid (10 mmol) and sodium hydroxide (10 mmol) was dissolved in a mixture of 10 ml of acetone / water (V / V, 1 / 1), and at room temperature (25 ° C) reaction for 10 minutes. Then, diulfide (15 mmol) was added. When carbon sulfur is added, the color of the solution is rapidly changed from colorless. The reaction was carried out after 2 h and then drip 2- (2- (2- (4-formylphenoxy) ethoxy) ethoxy) ethyl 2-bromopropane (10 mmol). Then 6 hours of reaction, then remove the acetone in the reaction with a rotary evaporation, and then extracted with ethyl acetate. The organic phase was dried with magnesium sulfate. After purification of column chromatography (ethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com