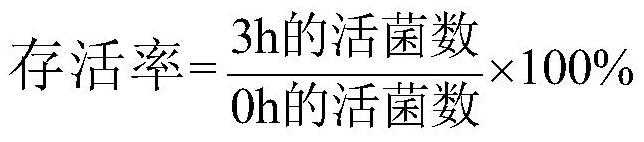A strain of Bifidobacterium breve and its cultivation method and use
A technology of Bifidobacterium breve and its cultivation method, which is applied in the field of Bifidobacterium breve and its cultivation, can solve the problems of dairy products and drug efficacy effects, gastric acid resistance and bile salt resistance, etc., and achieve low potential risk, Good resistance to gastric acid and bile salts, and the effect of reducing risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
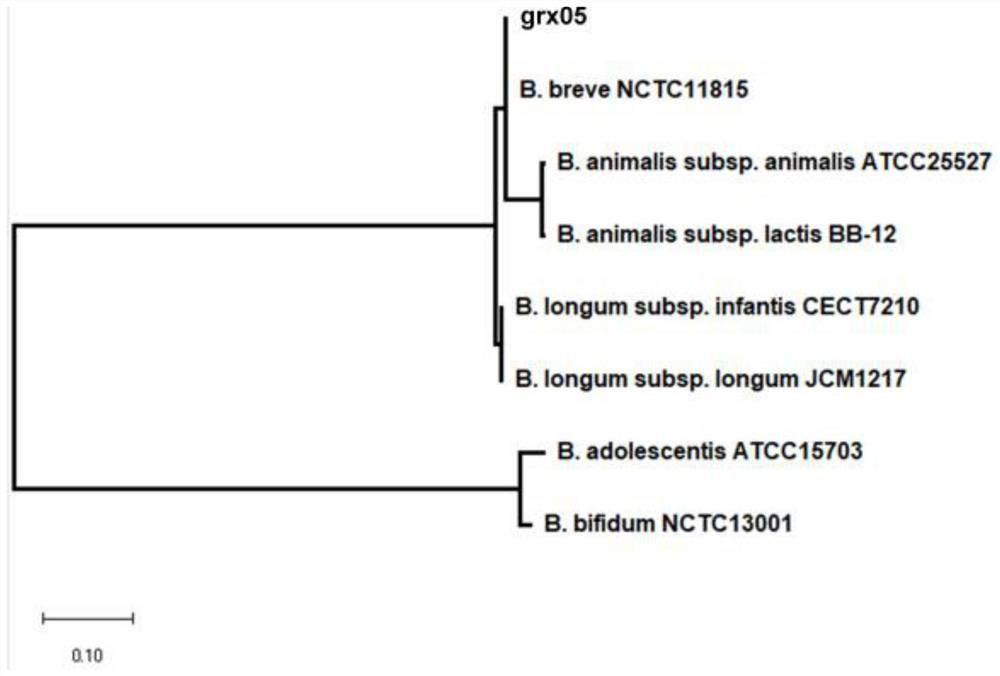
[0031] Example 1: Screening of strains
[0032] 1. Sample collection
[0033] Before sample collection, the nipples of healthy volunteers were subjected to strict sanitation treatment, and then about 10 mL of breast milk was collected and placed in sterile centrifuge tubes, and then stored in a -80°C ultra-low temperature refrigerator.
[0034] 2. Bacteria isolation
[0035] 1) Primary screening of strains
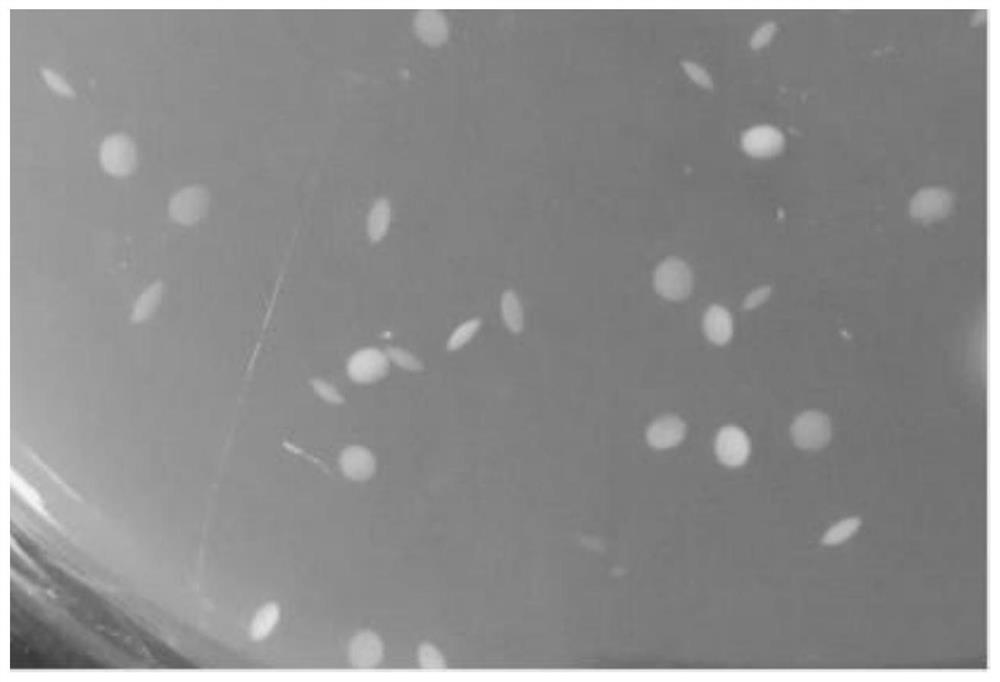
[0036] The breast milk samples after enrichment culture and direct gradient dilution were poured into MRS-Y1 and MRS-Y2 liquid medium, respectively, and cultured in anaerobic bags at 37 °C for 24-48 h until a single colony was grown. The typical morphology of Fidobacteria is a characteristic single colony of white or milky white.
[0037] 2) Strain rescreening
[0038] The strains obtained from the primary screening were streaked in MRS-G1 and MRS-G2 solid medium containing bromocresol violet, and cultured anaerobic for 48 hours. The selected strains with typical bifi...
Embodiment 2
[0064] Example 2: Acid and bile salt tolerance of Bifidobacterium breve grx05
[0065] 1) Test of acid resistance
[0066] Preparation of bacterial suspension: After 24 strains were screened and activated by anaerobic culture, they were respectively inoculated into MRS-Y1 liquid medium (cultivating bifidobacteria) and MRS-Y2 liquid medium ( cultured Lactobacillus), after anaerobic cultivation at 37°C for 24 hours, centrifuged at 6000 × g for 10 min to collect the bacterial cells, and sterilized PBS buffer was added to the bacterial cells to obtain a bacterial suspension, wherein the OD in the bacterial suspension 600 is 1.0.
[0067] Preparation of gastric juice: Dissolve 0.5g NaCl and 0.3g pepsin in deionized water respectively, adjust the pH to 3.0 with 1.0mol / L hydrochloric acid, add deionized water to 100mL, filter and sterilize with a 0.22μm filter, and refrigerate at 4°C spare.
[0068] Take 1.0 mL of bacterial suspension and inoculate it into 9.0 mL of gastric juice ...
Embodiment 3
[0082] Example 3: Inhibitory effect of strain on pathogenic bacteria
[0083] Six pathogenic bacteria, Escherichia coli, Staphylococcus aureus, Bacillus cereus, Bacillus subtilis, Salmonella and Pseudomonas were selected as indicator bacteria, and the Oxford cup method was used to determine the inhibitory effect of the strain on the pathogenic bacteria. shown in Table 4.
[0084] Pour thin-layer agar at the bottom of the plate, add the strains isolated in the embodiment of the present invention to the agar; culture the pathogenic bacteria in LB liquid medium at 37°C for 24 hours, and take 0.1 mL of the pathogenic bacteria liquid and spread it on 30 mL of LB. The surface of the solid medium; evenly place a sterilized Oxford cup in a horizontal plate, and draw 0.2 mL of the bacterial suspension to be tested into the Oxford cup. The plate was placed in a refrigerator at 3-4°C for 24h, and then incubated at 37°C for 24h, and the size of the inhibition zone was measured. Repeat th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com