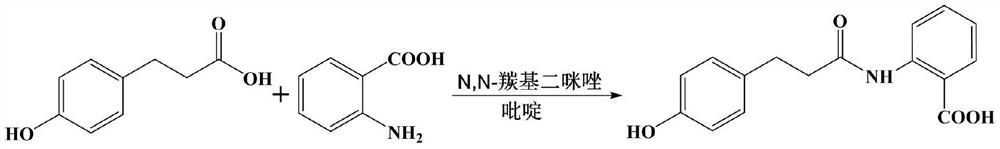
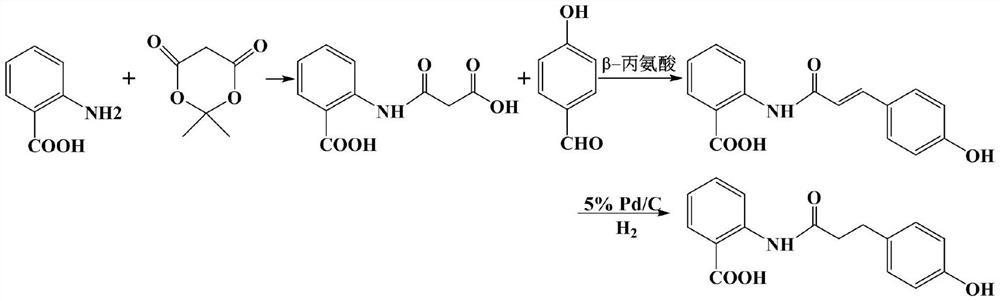
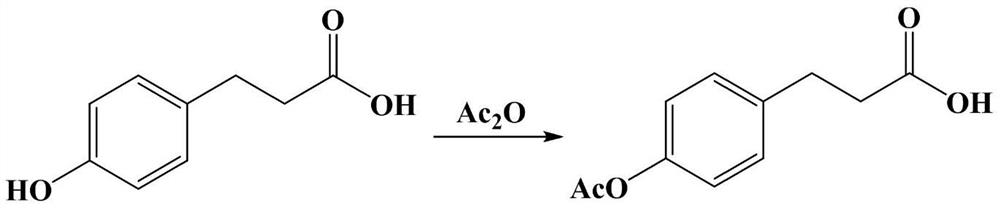
Preparation process of dihydrooat alkaloid D
An avenantha alkaloid and a preparation process technology, applied in the field of cosmetic chemistry, can solve the problems of difficulty in recovering and applying thionyl chloride, not easy to promote in industrialization, increase production and processing costs, etc., so as to reduce production and processing costs, inhibit hydrolysis, and reduce emissions. amount of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation technology of dihydrooat alkaloid D comprises the steps:
[0047] (1) Preparation of p-acetoxyphenylpropionic acid
[0048] Add 105 g (2.625 mol) of sodium hydroxide into a 5 L reactor, stir and dissolve in 320 mL of water, add 166 g (1 mol) of p-hydroxyphenylpropionic acid, and stir to dissolve. 224 g of acetic anhydride (2.2 mol) was added dropwise in an ice bath (-5-0°C). During the dropwise addition, the solution was a milky white suspension, and stirred at room temperature for 14 hours. The pH of the solution was adjusted to 4.4 with hydrochloric acid, extracted with ethyl acetate, washed with water, and dried to obtain 204.1 g of crude p-acetoxyphenylpropionic acid.
[0049] The above crude p-acetoxyphenylpropionic acid was dissolved in 150mL of toluene, connected to the water separator, azeotropic distillation for 2h, 120mL of toluene was distilled off, recycled, and dried to obtain 199.2g of p-acetoxyphenylpropionic acid, with a yield of 95.7%. ...
Embodiment 2
[0066] The preparation technology of dihydrooat alkaloid D comprises the steps:
[0067] (1) Preparation of p-acetoxyphenylpropionic acid
[0068] Add 100.8g (2.52mol) of sodium hydroxide into a 5L reactor, stir and dissolve in 320mL of water, add 166g (1mol) of p-hydroxyphenylpropionic acid, stir and dissolve. 214.4 g of acetic anhydride (2.1 mol) was added dropwise in an ice bath (-5-0°C). During the dropwise addition, the solution was a milky white suspension, and stirred at room temperature for 14 hours. The pH of the solution was adjusted to 4.3 with hydrochloric acid, extracted with ethyl acetate, washed with water, and dried to obtain 203.5 g of crude p-acetoxyphenylpropionic acid.
[0069] The above crude p-acetoxyphenylpropionic acid was dissolved in 150mL of toluene, connected to the water separator, azeotropic distillation for 2h, 115mL of toluene was distilled off, recycled, and dried to obtain 199.7g of p-acetoxyphenylpropionic acid, with a yield of 96.0%.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com