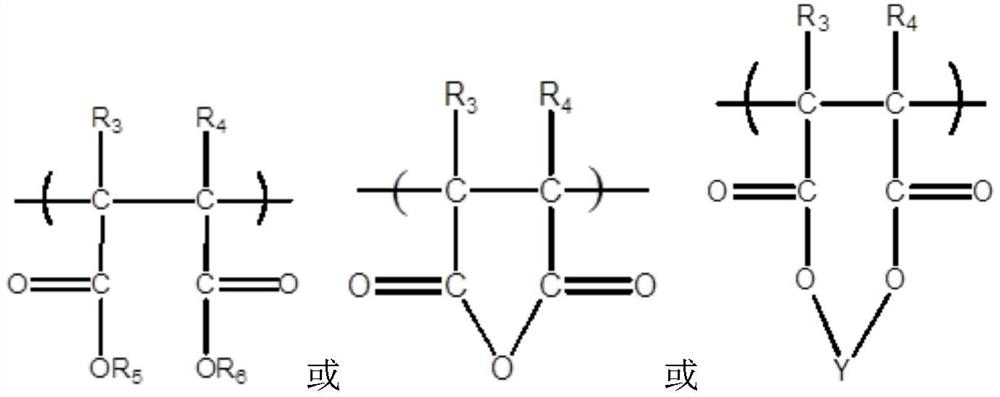
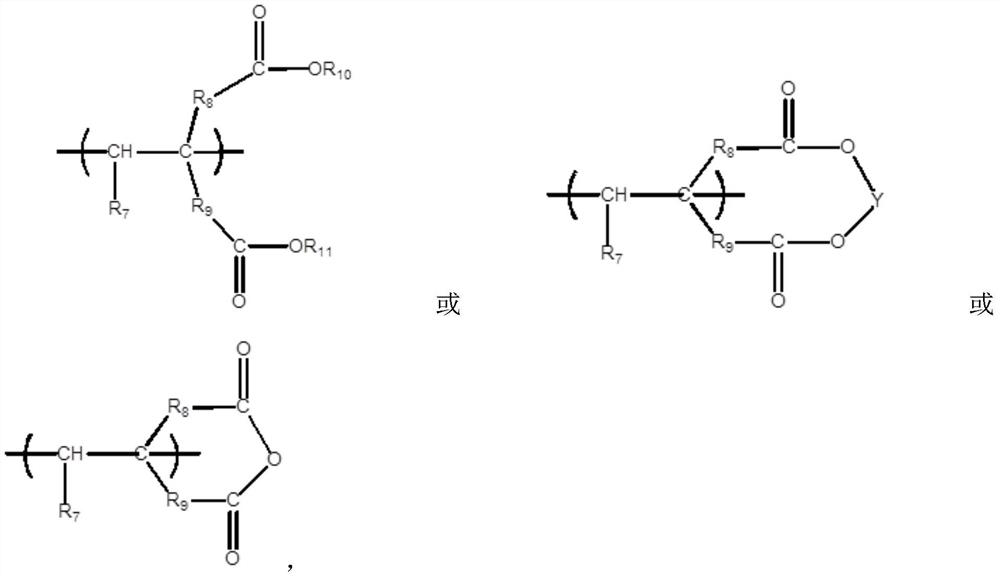
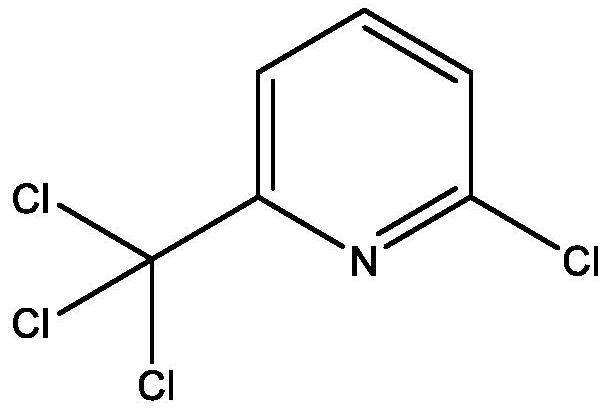
Nitrapyrin compositions for enhancing nitrogen nutrient use efficiency and improving plant growth
A technology of trichloromethylpyridine and composition, applied in the field of trichloromethylpyridine composition for improving nitrogen utilization efficiency and improving plant growth, can solve problems such as air loss, loss of product efficacy, reduction in efficiency, and the like, Achieve the effect of improving plant growth, reducing nitrification and reducing atmospheric ammonia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0264] It should be understood that the following examples are provided by way of illustration only and nothing therein should be considered limiting.
example 1
[0265] Example 1. Reducing the Volatility of Trichloromethylpyridine / Polyanionic Polymer Salts.
[0266] The three materials were placed on a thermal balance at 100° C. and their weights were observed every minute for 10 minutes as a percentage of the original weight. The materials are:
[0267] 1. Pure trichloromethylpyridine,
[0268] 2.25% w / w trichloromethylpyridine solution in sulfolane, and
[0269] 3. 25% solution of chloridinium salt with equimolar amount (acid based) of maleic acid-itaconic acid copolymer also in sulfolane (trichloromethylpyridine / polyanionic polymer salt).
[0270] The weight loss data in Tables 2-3 show that polyanionic polymer salts of trichloropicoline, both as a solution and as free trichloropicoline, exhibit Significantly reduced trichloromethylpyridine loss rate. The data show that trichloromethylpyridine volatilization was reduced by 10%-30% when used in salt form compared to untreated. Thus, the disclosed trichloromethylpyridinium salts ...
example 2
[0278] Example 2. Formation of Trichloromethylpyridinium Salt Solution and Fractionation of Solution.
[0279] Table 4: Trichloropicolin Salt Solutions Containing 20% Trichloropicolin
[0280]
[0281]
[0282] *Dipropylene Glycol, Dow PT250, Dow PT700, Triethylene Glycol, Tripropylene Glycol, Propylene Carbonate, Triacetin, Agnique AMD810, Agnique AMD3L, Rhodiasolv ADMA10, Rhodiasolv ADMA810, RhodiasolvPolarclean. Solvents that can be used to dissolve trichloropyridinium salts include, but are not limited to: aromatic solvents, especially alkyl-substituted benzenes, such as xylene or propylbenzene fractions, and mixed naphthalene and alkylnaphthalene fractions; mineral oil; kerosene ; fatty acid dialkylamides, especially fatty acid dimethylamides, such as caprylic acid dimethylamide; chlorinated aliphatic and aromatic hydrocarbons, such as 1,1,1-trichloroethane and chlorobenzene, diol derivatives esters of diethylene glycol n-butyl, ethyl or methyl ether acetate and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chemical stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com