Super-hydrophobic covalent organic framework material as well as preparation method and application thereof
A covalent organic framework, super-hydrophobic technology, applied in separation methods, textiles and papermaking, liquid separation, etc., can solve the problems that hinder the practical application of oil-water separation, loss of surface super-hydrophobicity, poor durability, etc., and achieve excellent oil-water separation stability The effect of high stability, simple preparation process and good practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
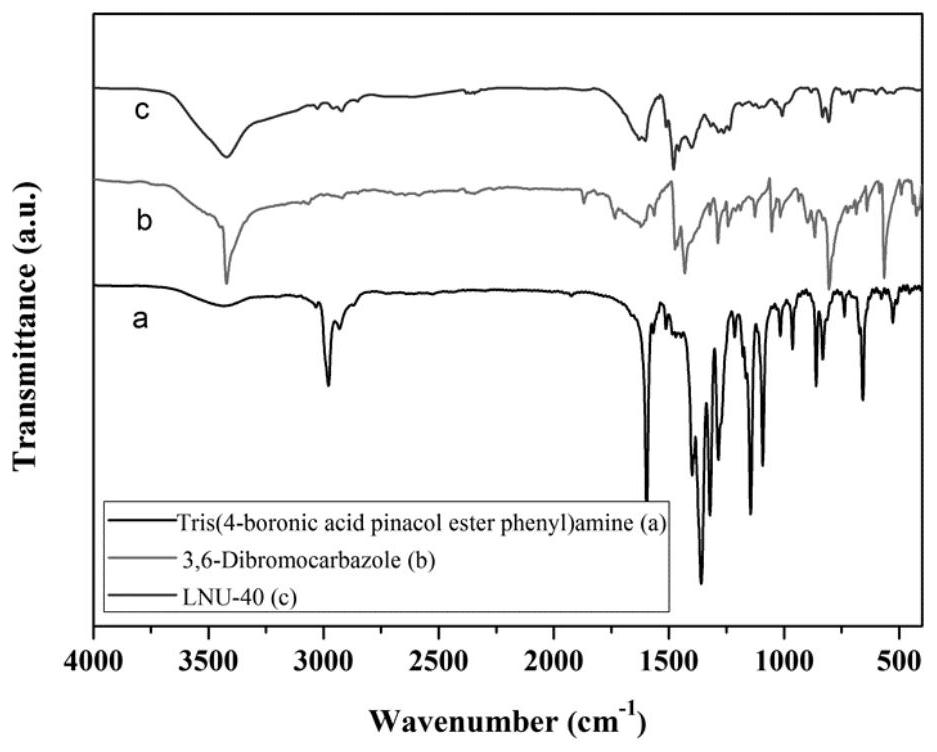
[0049] Example 1 Preparation of a superhydrophobic covalent organic framework material LNU-40
[0050] 1. Synthesis of LNU-40
[0051] Add 400mg of tris(4-boronic acid pinesol ester phenyl)amine (0.6418mmol) and 312.89mg of 3,6-dibromocarbazole (0.96274mmol) into 60mL N,N'-dimethylformamide for dissolution, set In a 100mL round-bottomed flask, use liquid nitrogen to freeze and use an oil pump to evacuate the nitrogen for three times. Add 80mg of tetrakis(triphenylphosphine)palladium and 5mL of potassium carbonate solution (2moL / L) to the reaction system quickly, and then repeat the cycle of desorption. The reaction mixture was heated to 130 °C and stirred and refluxed for 48 hours under nitrogen protection.
[0052] 2. Post-processing of LNU-40
[0053] Suction filter the reactant to leave solid insoluble matter, which is washed with tetrahydrofuran, water and acetone solvent several times to remove possible residual unreacted monomer or catalyst residue. The crude product ...
Embodiment 2
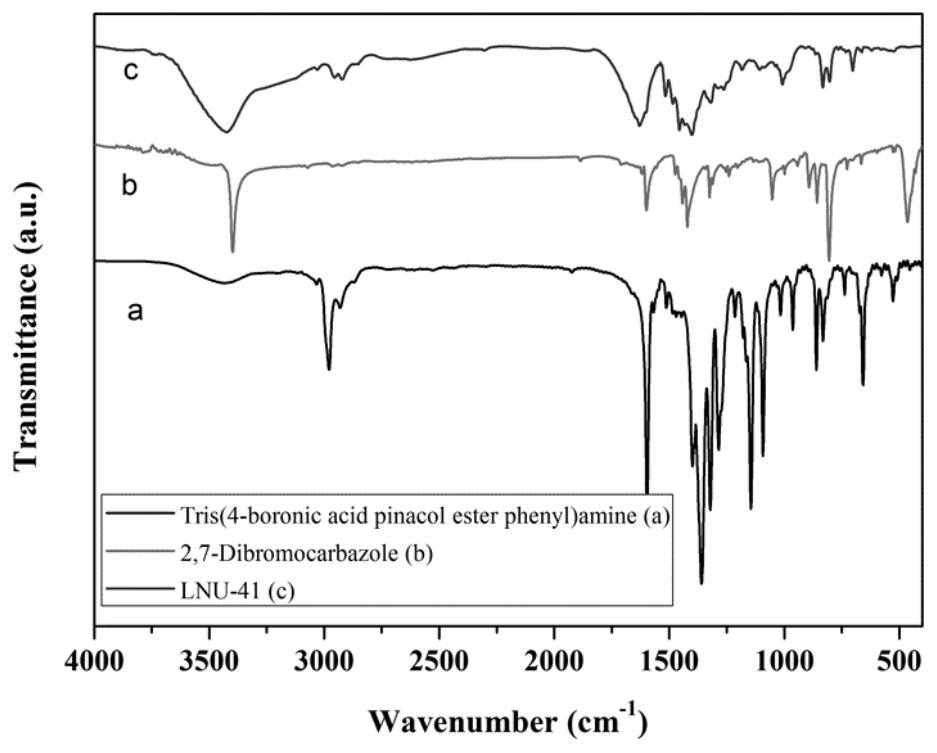
[0055] Example 2 Preparation of a superhydrophobic covalent organic framework material LNU-41
[0056] 1. Synthesis of LNU-41
[0057] 400 mg of tris(4-boronic acid pinesol ester phenyl)amine (0.6418 mmol) and 312.89 mg of 2,7-dibromocarbazole (0.96274 mmol) were dissolved in 60 mL of N,N'-dimethylformamide, Place in a 100mL round-bottomed flask, freeze with liquid nitrogen and evacuate nitrogen with an oil pump for three times, add 80mg tetrakis(triphenylphosphine) palladium and 5mL potassium carbonate solution (2moL / L) to the reaction system quickly, and then repeat the cycle After degassing, the reaction mixture was heated to 130°C and stirred at reflux for 48 hours under nitrogen protection.
[0058] 2. Post-processing of LNU-41
[0059] Suction filter the reactant to leave solid insoluble matter, which is washed with tetrahydrofuran, water and acetone solvent several times to remove possible residual unreacted monomer or catalyst residue. The crude product was further ...
Embodiment 3
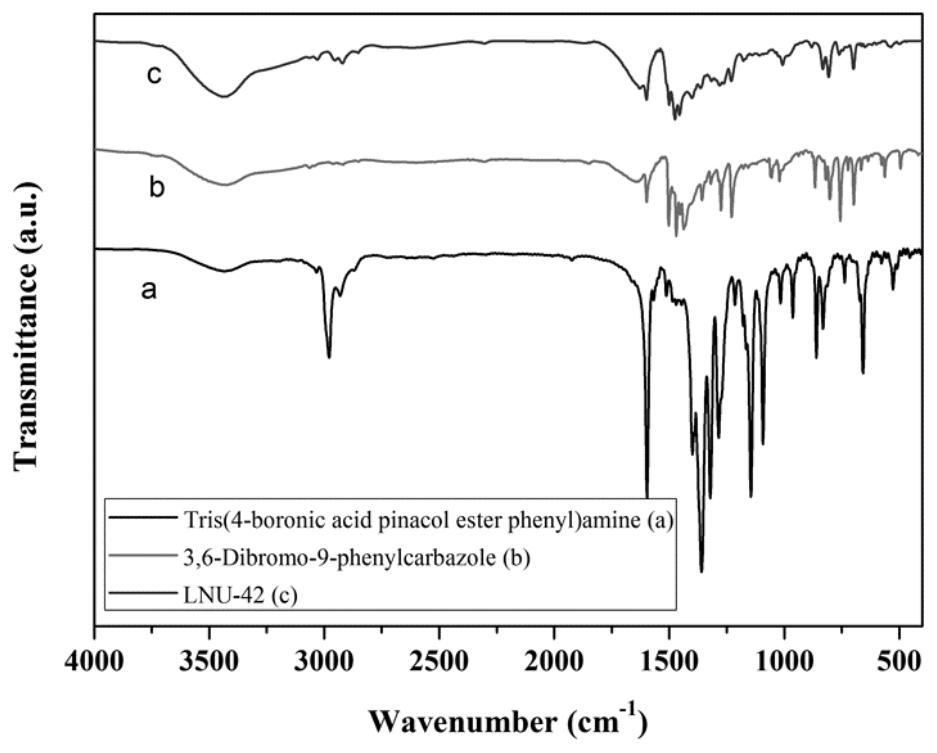
[0061] Example 3 Preparation of a superhydrophobic covalent organic framework material LNU-42
[0062] 1. Synthesis of LNU-42
[0063] Add 400mg of tris(4-boronic acid pinesol ester phenyl)amine (0.6418mmol) and 386.16mg of 3,6-dibromo-9-phenylcarbazole (0.96274mmol), add 60mL N,N'-di Methylformamide was dissolved, placed in a 100mL round-bottomed flask, frozen with liquid nitrogen and evacuated with an oil pump to vent nitrogen three times, and 80mg of tetrakis(triphenylphosphine)palladium and 5mL of potassium carbonate solution (2moL / L) were quickly added to the reaction system, and then repeated cycle degassing, the reaction mixture was heated to 130 ° C and stirred and refluxed under nitrogen protection conditions for 48 hours.
[0064] 2. Post-processing of LNU-42
[0065] Suction filter the reactant to leave solid insoluble matter, which is washed with tetrahydrofuran, water and acetone solvent several times to remove possible residual unreacted monomer or catalyst res...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water contact angle | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com