Antibacterial organic compound as well as preparation method and application thereof
A technology of organic compounds and antibacterial agents, which is applied in the preparation of organic compounds, hydroxyl compounds, and nitro compounds, etc., can solve problems such as the increase in the number of multidrug-resistant strains, and achieve long-term antibacterial ability, significant effect, and reduced The effect of the speed of resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
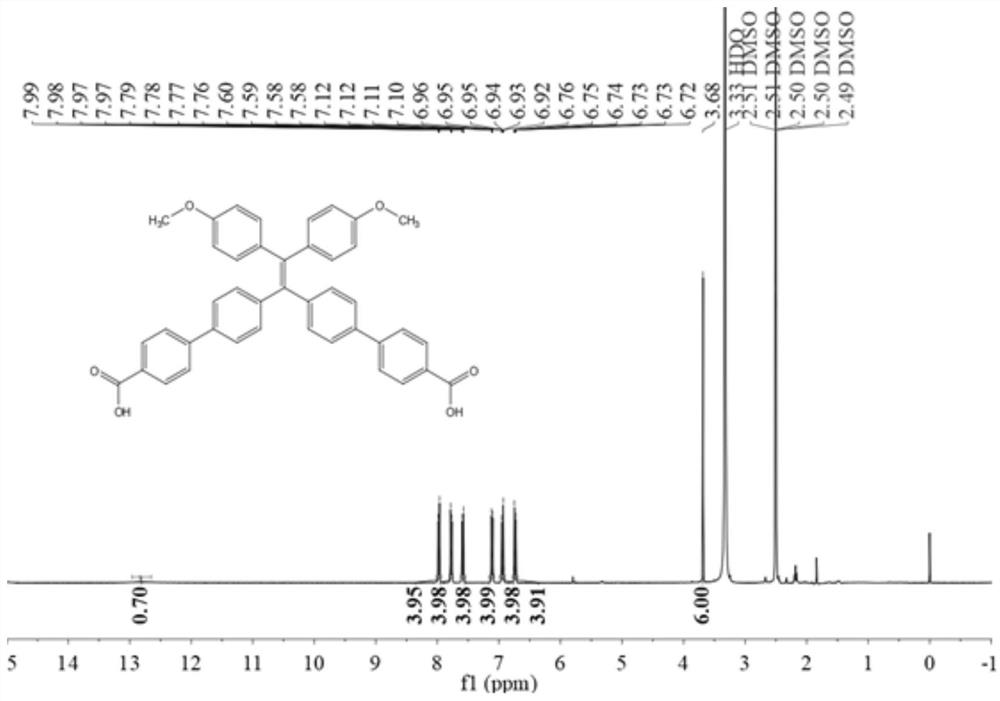
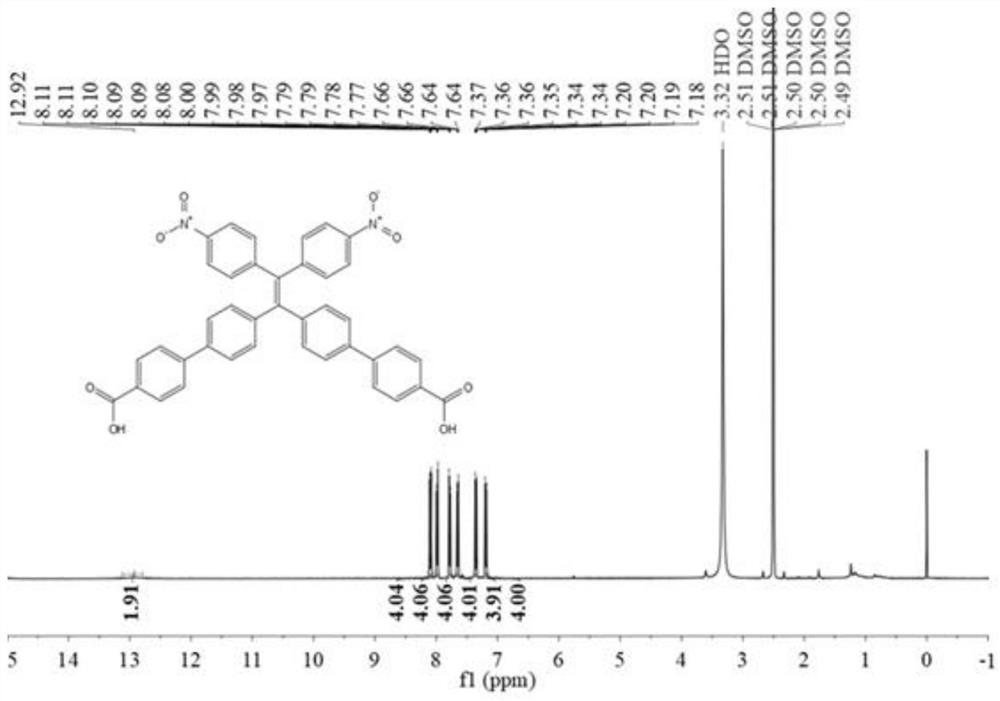
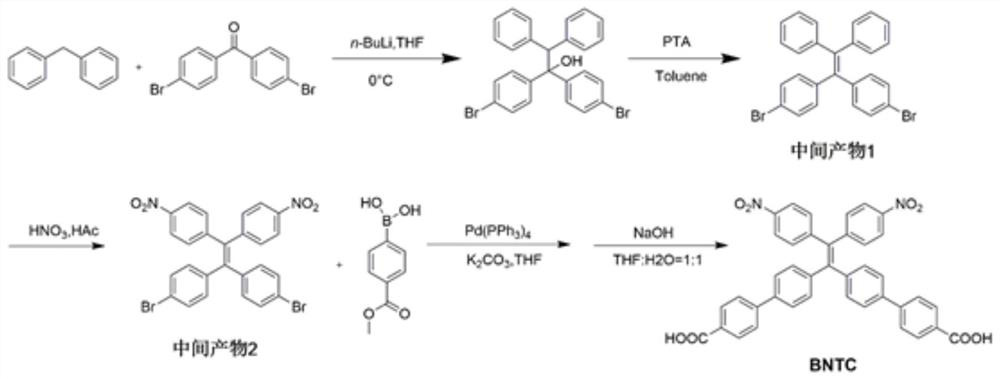
[0065] Embodiment 1: the synthesis of BNTC
[0066] The synthetic route is attached figure 1 .
[0067] In a nitrogen atmosphere, at 0 ° C, to a solution containing diphenylmethane (2.5 g, 15 mmol) in anhydrous tetrahydrofuran (40 mL) was added 9 mL of a 1.6 M n-butyllithium solution in hexane, at which temperature the obtained The orange-red solution was stirred for 30 minutes. After addition of 4,4'-dibromobenzophenone (5.1 g, 15 mmol), the reaction mixture was allowed to warm to room temperature and stirring was continued for 6 hours. Afterwards, the reaction mixture was quenched with aqueous ammonium chloride solution, followed by multiple extractions with dichloromethane. The organic layer was dried over anhydrous sodium sulfate, and the crude alcohol product was obtained by evaporating the solvent. The obtained crude alcohol product was dissolved in about 100 ml of toluene, and p-toluenesulfonic acid (1.15 g, 6.0 mmol) was added, and the reaction mixture was refluxed...
Embodiment 2
[0071] Embodiment 2: the synthesis of BOTC
[0072] The synthetic route is attached Figure 4 .
[0073] 4,4'-dibromobenzophenone (3.91g, 11.5mmol), 4,4'-dimethoxybenzophenone (2.79g, 11.5mmol) and zinc powder (5.97g, 92mmol) were dissolved in dehydrated tetrahydrofuran, then the mixture was purged with nitrogen. Titanium tetrachloride (8.72 g, 46 mmol) was added to the above mixture under nitrogen protection, and the acetone bath was maintained for 0.5 hours. The mixture was then refluxed under nitrogen for 9 hours and then cooled to room temperature. After adding saturated potassium carbonate solution, it was extracted three times with dichloromethane. The organic phase was dried with anhydrous sodium sulfate to obtain a crude product, which was purified on a silica gel column using petroleum ether / dichloromethane (5 / 1v / v) as the eluent to obtain intermediate product 3 as a light yellow powder (Yield 35%).
[0074] Intermediate 3 (550mg, 1.0mmol), 4-methoxycarbonylphen...
Embodiment 3
[0076] Embodiment 3: the preparation of magnesium aluminum ultrathin hydrotalcite nanosheet (LDH)
[0077] Solution A: Mg(NO 3 ) 2 ·6H 2 O(0.4mmol), Al(NO 3 ) 3 9H 2 O (0.2 mmol) was dissolved in 20 mL of deionized water.
[0078] Solution B: NaNO 3 (0.2 mmol) was dissolved in 40 mL of deionized water containing 25% formamide.
[0079] Solution C: NaOH (4.5 mmol) was dissolved in 20 mL of deionized water. Add solution A and solution C to solution B rapidly at 80°C, and after stirring vigorously for 20 minutes, wash the obtained hydrotalcite with water and ethanol several times to neutrality, then perform dialysis (3kDa) to remove formamide, and finally in Resuspend in water for later use.
[0080] as attached Figure 7 , the ultra-thin hydrotalcite was characterized by transmission electron microscopy, the prepared ultra-thin hydrotalcite is a sheet structure, the particle size is about 70nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com