Preparation method and application of cadmium sulfide quantum dots
A technology of quantum dots and cadmium sulfide, applied in the field of preparation of cadmium sulfide quantum dots, can solve the problems of low detection sensitivity, high reaction temperature, and harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The present invention also provides a preparation method for the above-mentioned cadmium sulfide quantum dots, specifically comprising the following steps:
[0041] Preparation containing CdCl 2 and a Cd / thiourea precursor solution of thiourea;
[0042] Mixing the Cd / thiourea precursor solution with a stabilizer and adjusting the pH to alkaline;
[0043] Add Na to the mixed solution adjusted to alkaline 2 S, mixed, that is.
[0044] Among them, CdCl in Cd / thiourea precursor liquid 2 The mass ratio to thiourea is (1.5~2.8):1, preferably (1.8~2.5):1, wherein, when controlling the CdCl in the Cd / thiourea precursor liquid 2 When the mass ratio of cadmium sulfide to thiourea is 2:1, the detection effect of the prepared cadmium sulfide quantum dots is the best. It should be noted that during the synthesis process, the dosage of thiourea needs to be strictly controlled. If there is too much or too little thiourea, the fluorescence intensity of the prepared quantum dots wi...
Embodiment 1
[0049] a. Mix 0.04g CdCl in 14ml ultrapure water 2 2.5H 2 O and 0.015g thiourea to form a Cd / thiourea precursor solution, add 38μL thioglycolic acid (TGA) to 20mL water, then mix the two, and use 1mol L -1 Adjust the pH of the mixed solution to 10.5 with NaOH, then add 0.005g, 0.01g, 0.02g, 0.03g, 0.04g, 0.06g, 0.08g, 0.1g Na 2 S·9H 2 0, stirred for 30min to obtain the synthesized CdS quantum dots.
[0050] b. Mix 0.04g CdCl in 14ml ultrapure water 2 2.5H 2 O and thiourea to form a Cd / thiourea precursor solution, add 38 μL thioglycolic acid (TGA) to 20 mL of water, then mix the two, and use 1mol L -1 Adjust the pH of the mixed solution to 10.5 with NaOH, then add 0.02g Na 2 S·9H 2 O, stirred for 30min to obtain the synthesized CdS quantum dots, wherein the amount of thiourea was 0.005g, 0.01g, 0.015g, 0.022g respectively.
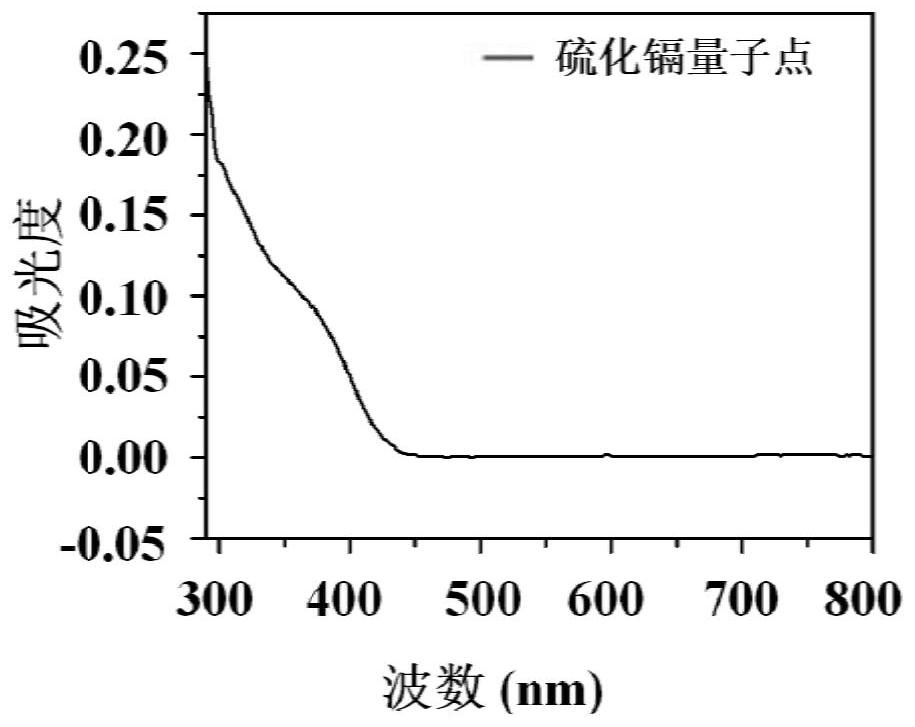
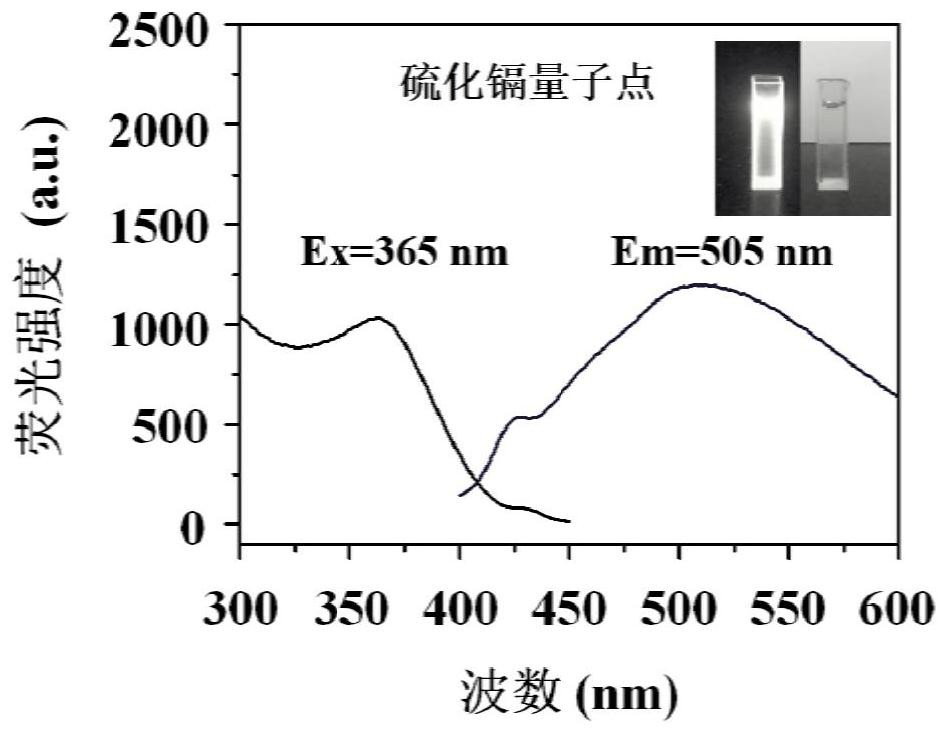
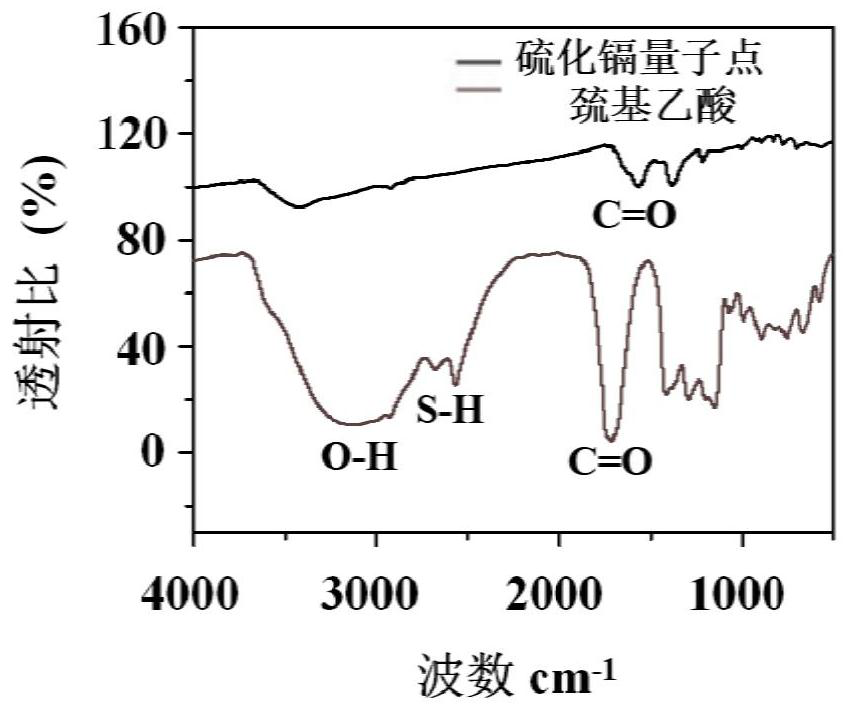
[0051] Characterize the CdS quantum dots synthesized in the two synthesis methods a and b in Example 1, and study its characteristics. Microscopy...
Embodiment 2
[0060] Example 2 Exploration of Factors Affecting Fluorescence Intensity of CdS QDs
[0061] (1) Effect of amino acid peptides on the fluorescence intensity of CdS QDs
[0062] Add 100 μL glycine (Gly), tyrosine (Tyr), aspartic acid (Asp), cystine (Cys-Cys), tryptophan (Try), L-cysteine to 50 μL CdS QDs solution Acid (Cys) (10 -5 mol mL -1 ) and 20 μL glutathione (GSH) (4×10 -5 mol mL -1 ) standard solution, dilute to 5.0 mL with double distilled water. Let stand at room temperature for 5 min, and record the fluorescence intensity (λex=365 nm) of the obtained solution with a fluorescence photometer. Test results such as Figure 7 As shown, the fluorescence intensity of CdSQDs was significantly enhanced by glutathione, and the fluorescence intensity of CdS QDs could be increased by 1.4 times in the presence of glutathione (GSH).
[0063] To further explore the effect of different concentrations of GSH on the fluorescence intensity of CdS QDs: 5, 10, 15, 20, 25, 62.5, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com