Preparation method of stable isotope labeled oxazepam internal standard reagent
A stable isotope and labeling technology, which is applied in the preparation of organic compounds, isotope introduction of heterocyclic compounds, and isotope introduction into organic compounds, etc., can solve the strict requirements of stable isotope labeling reagent conditions, low total yield of final products, and technical difficulties. Large problems such as short synthesis steps, easy availability of raw materials, and high chemical purity of products are achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
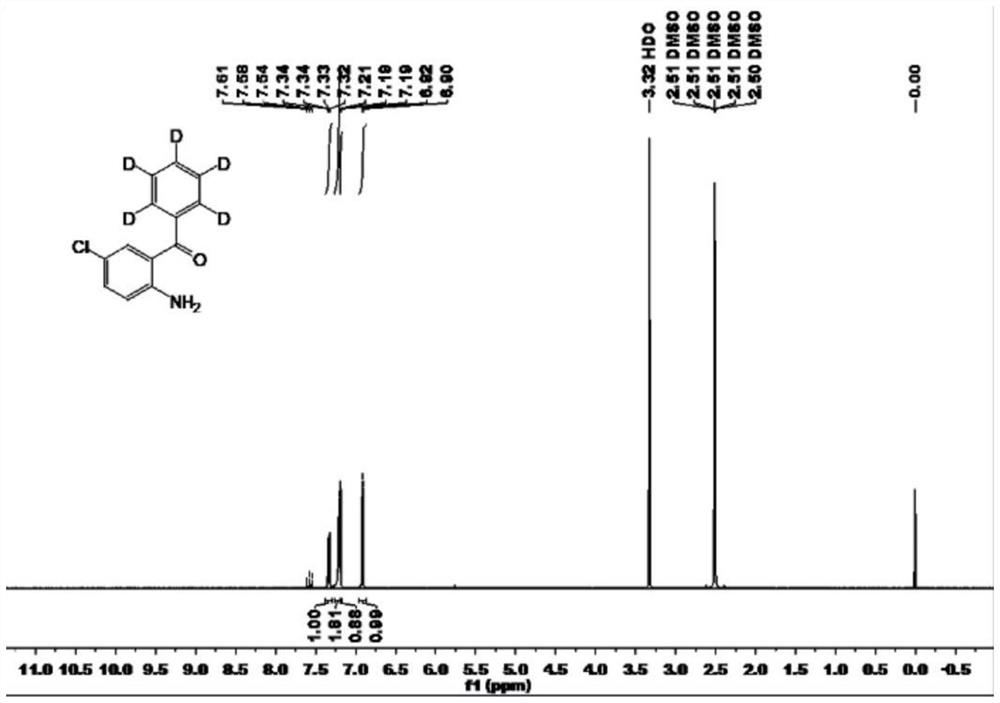
[0048] Embodiment 1: 2-amino-5-chlorobenzophenone (phenyl-D 5 )preparation
[0049]
[0050] In a 500mL three-necked flask, add deuterated phenylboronic acid (12.7g, 0.1mol), 2-amino-5-chlorobenzophenone (7.63g, 0.05mol), catalyst tetrakis (triphenylphosphine) palladium (2.89g , 2.5mmol), ligand 4,4'-dimethyl-2,2'-bipyridine (920mg, 5mmol), additive trifluoroacetic acid (57g, 0.5mol) and tetrahydrofuran: water = 1:1 mixed solvent (250mL); reacted at 90°C for 24 hours; after TLC traced the reaction raw materials to complete the reaction, the reaction liquid was cooled to room temperature, 200mL of water was added, extracted with ethyl acetate (3×300mL), the combined organic phases were washed with saturated bicarbonate successively Washing with sodium solution, washing with saturated sodium chloride solution, adding anhydrous sodium sulfate to dry, distilling off the solvent under reduced pressure, and obtaining the stable isotope labeled 2-amino-5-chlorobenzophenone (pheny...
Embodiment 2
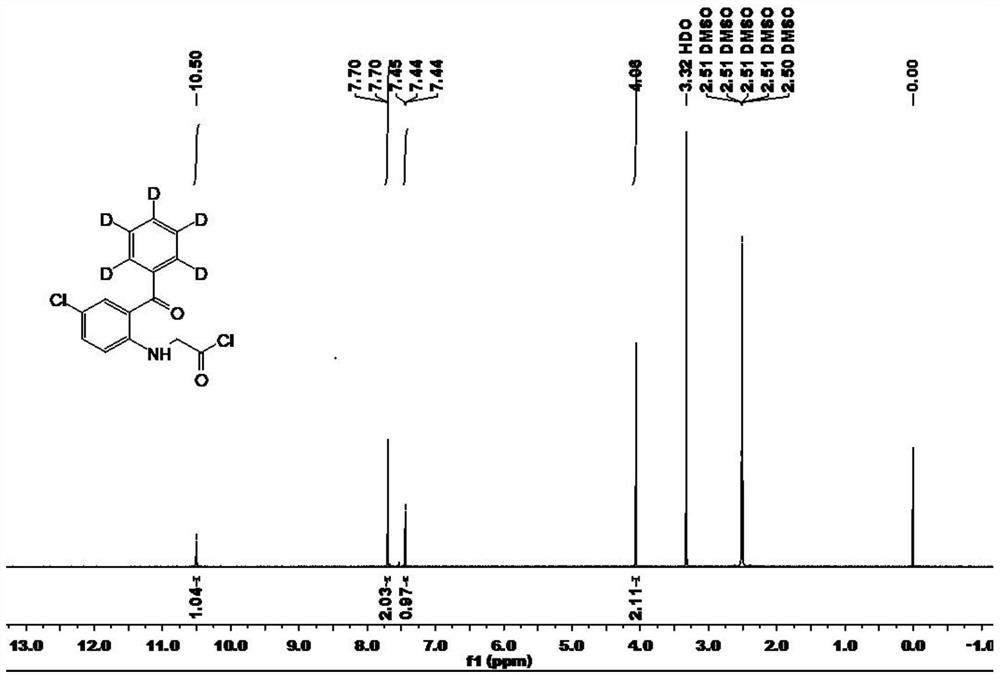
[0051] Example 2: 2-chloroacetamido-5-chlorobenzophenone (phenyl-D 5 ) preparation
[0052]
[0053] In a 100mL three-necked flask, add 2-amino-5-chlorobenzophenone (phenyl-D 5 ) (2.37g, 10mmol), chloroacetyl chloride (1.69g, 15mmol) and dry dioxane (20mL); filled with nitrogen, reacted for 12 hours at 20 ° C; Remove the solvent by distillation under pressure, add 20mL of water, extract with ethyl acetate (3×30mL), combine the organic phases, wash with saturated sodium bicarbonate solution and saturated sodium chloride solution successively, add anhydrous sodium sulfate to dry, and distill under reduced pressure Remove the solvent, and the residue obtains stable isotope labeling 2-chloroacetamido-5-chlorobenzophenone (phenyl-D 5 ) (2.97g, yield 95%). 1 H NMR (DMSO-D 6 , 600MHz): δ10.50(s, 1H), 7.70(d, 2H), 7.44(t, 1H), 4.06(s, 1H), see attached image 3 .
Embodiment 3
[0054] Example 3: 7-chloro-1,3-dihydro-5-phenyl-2H-1,4-benzodiazepine -2-keto (phenyl-D 5 ) preparation
[0055]
[0056] In a 50mL three-neck flask, add stable isotope labeled 2-chloroacetamido-5-chlorobenzophenone (phenyl-D 5 ) (3.13g, 10mmol), urotropine (2.80g, 20mmol), ammonium chloride (1.07g, 20mmol), methanol (20mL); under nitrogen protection, heated to reflux, and reacted at this temperature for 24 hours After TLC traces the reaction raw material to react completely, remove solvent by distillation under reduced pressure, add 20mL water, extract with ethyl acetate (3 * 30mL), combine organic phase, wash with saturated sodium bicarbonate solution successively, saturated sodium chloride solution washes, Add anhydrous sodium sulfate to dry, distill off the solvent under reduced pressure, and the residue is subjected to column chromatography to obtain stable isotope labeled 7-chloro-1,3-dihydro-5-phenyl-2H-1,4-benzodiazepine -2-keto (phenyl-D 5 ) (2.97g, yield 95%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com