Antibacterial and antiviral porcine hemoglobin beta chain C-terminal fragment, bacillus subtilis expressing fragment, preparation and application
A technology of Bacillus subtilis and porcine hemoglobin, applied in Bacillus subtilis and its application fields, can solve the problems of poor stability of porcine hemoglobin peptide structure, physical and chemical properties and functional properties, poor antibacterial and antiviral performance, and reduced activity, and achieve good secretion , good non-pathogenicity, growth-inhibiting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Determination of the C-terminal Fragment of Porcine Hemoglobin β Chain with Antibacterial and Antiviral Dual Activities
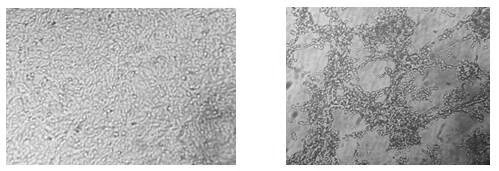
[0037] Using porcine hemoglobin β chain as template, from N- end to C- The end is gradually decomposed into fragments with a size of 15-17 amino acids. The two connected fragments overlap by 5 amino acids. A total of 14 polypeptide fragments are designed. See the design intention figure 1 . Changzhou Xiangtai Biotechnology Co., Ltd. was commissioned to use automated peptide synthesis equipment to efficiently and quickly synthesize the designed 14 peptide fragments, which were recorded as fragment 1 to fragment 14. See Table 1.
[0038] Table 1 Sequences of 14 polypeptide fragments
[0039]
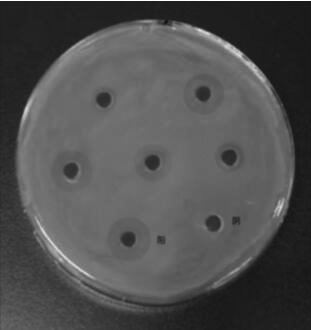
[0040] 1.1 Determination of antiviral activity of polypeptide fragments according to cytopathic inhibition method
[0041] Use the PEDV / Vero system to detect the biological activity of the polypeptide fragments. The Vero cell suspension digeste...
Embodiment 2
[0055] Example 2 High expression of porcine hemoglobin β chain C-terminal fragment in Bacillus subtilis
[0056] 2.1 Construction of pHT01-P43-HBB expression vector
[0057] Respectively according to the restriction site BamH I nucleic acid artificial sequence (sequence 1), Bacillus subtilis P43 constitutive promoter nucleic acid artificial sequence (sequence 2), porcine hemoglobin β chain C-terminal fragment nucleic acid artificial sequence [abbreviation: HBB] (sequence 3 ), the enzyme cutting site Sma I nucleic acid artificial sequence (sequence 4) entrusted Sangon Bioengineering (Shanghai) Co., Ltd. to synthesize its entire expression cassette (sequence 5) and insert it into the Bacillus subtilis expression vector pHT01 to obtain pHT01-P43- HBB recombinant vector, see Figure 4 .
[0058] The above sequences 1, 2, 3, 4 are sequentially connected in series.
[0059] 2.2 Competent preparation of Bacillus subtilis
[0060] Pick a single colony of B. subtilis WB800n on the ...
Embodiment example 3
[0070] Implementation Case 3 Production of high-density fermentation of Bacillus subtilis recombinant bacteria producing porcine hemoglobin β chain C-terminal fragment
[0071]The recombinant strain of Bacillus subtilis producing the C-terminal fragment of the porcine hemoglobin β chain was streaked on an LB plate containing 30 µg / mL of chloramphenicol resistance, and a single colony was selected and inoculated into a 1L Erlenmeyer flask containing 250 mL of LB liquid medium. Cultivate in a shaker at 37°C and 200rpm until OD600=0.6-0.8; after microscopic examination without bacteria, it is used as the first-class seed for later use. A total of 1 L of 4 bottles of first-grade seed liquid was added to 100 L of sterilized fermentation medium. During the fermentation process, the temperature was maintained at 37 °C and the pH value was maintained at 8.0 (the pH value was maintained by dropping 10% ammonia water), and The dissolved oxygen (dissolved oxygen, DO) was maintained at 20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com