Beta-nitroketone as well as preparation method and application method thereof
A technology for nitroketones and nitrohydrocarbons, applied in the field of organic chemical synthesis, which can solve the problems of difficult separation and purification and low yield of nitrohydrocarbons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
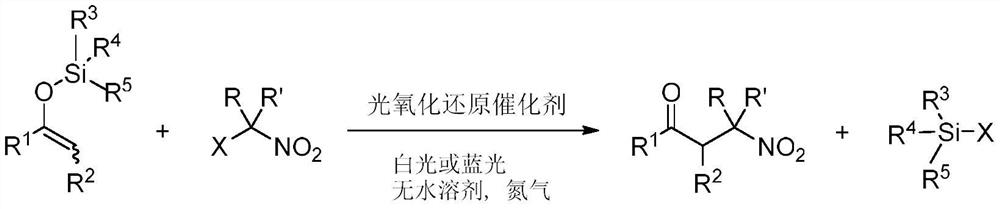
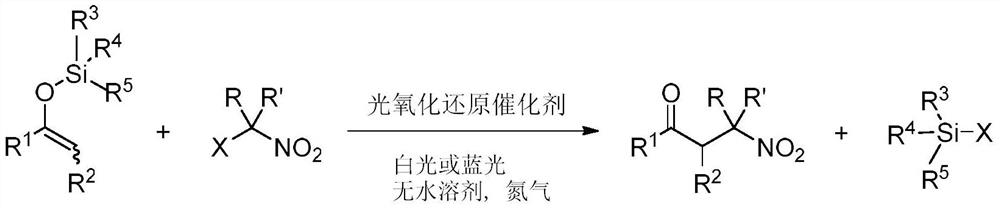
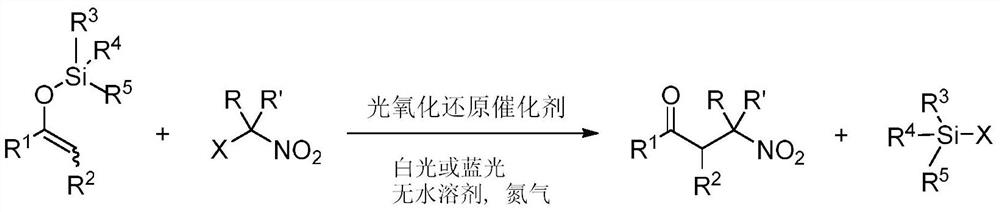
[0022] This embodiment provides a method of preparation of β-nitrosyl ketone, including the following preparation step: as an enol silicone ether and a lazal nitro-hydrocarbon, under anhydrous solvent, a photoxidation reduction catalyst, at room temperature, The stirring reaction was stirred under visible light, and the reaction liquid was purified by purification of β-nitrone, and its reaction formula is as follows:
[0023]
[0024] In one example, in the above enol silica ether, R 1 R 2 The same or different groups can be selected independently. For example, R 1 From one of the following: C1 to C10 alkyl, C2 to C10 unsaturated hydrocarbon group, C3 to C10 of cycloalkyl, aryl; R 2 From one of the following: alkyl group of hydrogen, C1 to C8. Example floor, when R 2 When the alkyl group from C1 to C8, the alkyl group of C1 to C8 may include methyl, ethyl, n-propyl, isopropyl, tert-butyl, pentyl, hexyl, heptyl, octyl, and the like. Rim 3 R 4 R 5 The alkyl group or phenyl groups ...
Embodiment 1
[0073] This example provides a method for preparing 3-methyl-3-nitro-1-phenyl-1-butanone, and the reaction formula is as follows:
[0074]
[0075] The preparation method includes the steps of: Take a completely dry Schlenk tube, vacuum nitrogen gas three times. 460 mg of 1-phenyl-1-trimethylsiloxyethylene (2.38 mmol), 200 mg of 2-bromo-2-nitropoxane (1.19 mmol) and 6.0 ml of anhydrous acetonitrile were added under nitrogen protection. 4mg FAC-IR (PPY) 3 (0.5 mol%). The reaction mixture was degassed by "frozen-vacuum-thaw" method, and then stirred under a 460 nm of 460 nm, 15W from the reaction tube, and the reaction temperature was controlled at 25 ° C, and the reaction was monitored by thin layer chromatography. After 2 hours, the reaction was completed. The concentrated reaction solution was rotationally evaporated to give a crude product. The crude product was purged with petroleum ether / ethyl acetate (volume ratio of 10: 1) rapid silica gel column chromatography to give a...
Embodiment 2
[0079] The reaction form is in the same embodiment. Visible photocatalyst FAC-IR (PPY) 3 The amount of amount was 8 mg (1.0 mol%), and the amount of other reagents was used as shown in Example 1.3-methyl-3-nitro-1-phenyl-1-butanone yield was 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com