Fusion protein and method for preparing semeglutide intermediate polypeptide from fusion protein
A technology of fusion protein and fusion peptide, which is applied in the field of genetic engineering and polypeptide preparation, can solve problems such as the inability to effectively increase the expression of fusion proteins, the lack of industrial scale-up value, and the lack of industrial scale-up significance, so as to improve extraction and enzymatic digestion. Efficiency, reduced dissolution loss, improved yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Construction of recombinant engineering bacteria expressing semaglutide intermediate polypeptide fusion protein
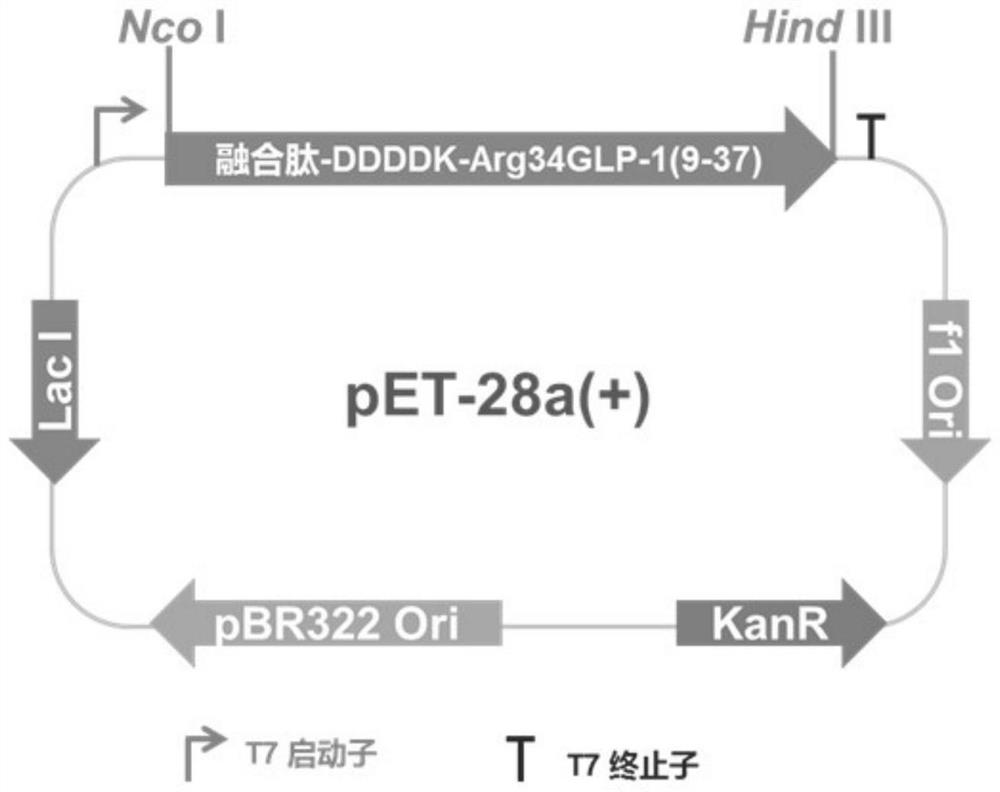
[0055] A fusion protein sequence was designed for expression in E. coli: fusion peptide-DDDDK-Arg34GLP-1(9-37).
[0056] The amino acid sequence of the fusion peptide can firstly enhance the expression, and secondly can protect the intermediate polypeptide Arg34GLP-1(9-37) from being degraded by Escherichia coli's own protease. The amino acid sequence of the fusion peptide is MATKAVSVLKGDGPVQGIINFEQKESNGPVKVWGSIKGLTEGLHGFHVHKFVNQHLCGSHLVALYLV (SEQ ID NO: 3). The C-terminus of the fusion peptide sequence is connected to the intermediate polypeptide Arg34GLP-1(9-37) of semaglutide through DDDDK residues, so the complete amino acid sequence of the fusion protein is MATKAVSVLKGDGPVQGIINFEQKESNGPVKVWGSIKGLTEGLHGFHVHKFVNQHLCGSHLVALYLV DDDDK EGTFTSDVSSYLEGQAAKEFIAWLVRGRG (SEQ ID NO: 12), the isoelectric point of this sequence is 6.2, and the average ...
Embodiment 2
[0058] Example 2: Expression of sameglutide intermediate polypeptide fusion protein in shake flask system
[0059] The recombinant engineered bacterium S1 obtained in Example 1 was cultured in LB medium at 37° C. for 12 hours to obtain a seed solution, which was then inserted into TB medium according to the inoculum size of 0.2% (v / v) for cultivation. When the fermentation broth OD 600 When the value reached 6-8, IPTG with a final concentration of 0.1 mM was added for induction, and the fermentation was terminated after 16 hours of induction at 37° C., and the bacteria were collected by centrifugation.
Embodiment 3
[0060] Example 3: Expression and expression level detection of sameglutide intermediate polypeptide fusion protein in shake flask system
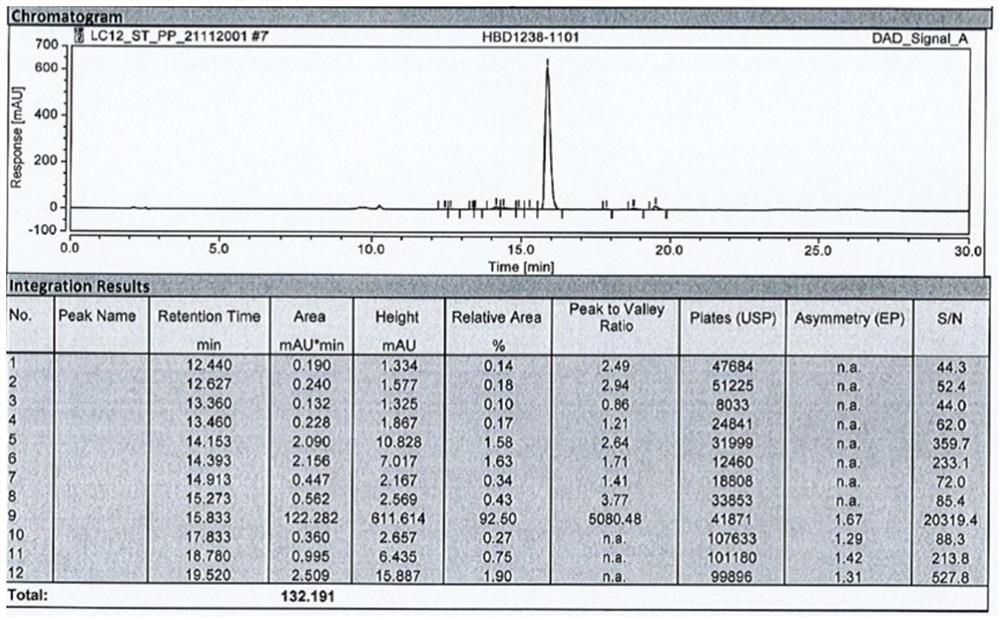
[0061] Wash the fermented cells obtained in Example 2, crush the cells with an ultrasonic breaker, and centrifuge the crushed suspension to collect inclusion bodies. SDS-PAGE was performed on the whole bacteria and inclusion bodies, and the electrophoretic purity of the target protein was detected by an optical densitometer. At the same time, the BCA kit was used to detect the total protein content of the whole bacteria and inclusion bodies. The expression amount of the intermediate polypeptide fusion protein was obtained by multiplying the total protein amount by the electrophoretic purity. After testing, recombinant engineering bacteria S1 was fermented and induced to express, and 1.56 g / L fusion protein was obtained, and 0.95 g / L inclusion bodies were obtained after crushing and washing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com