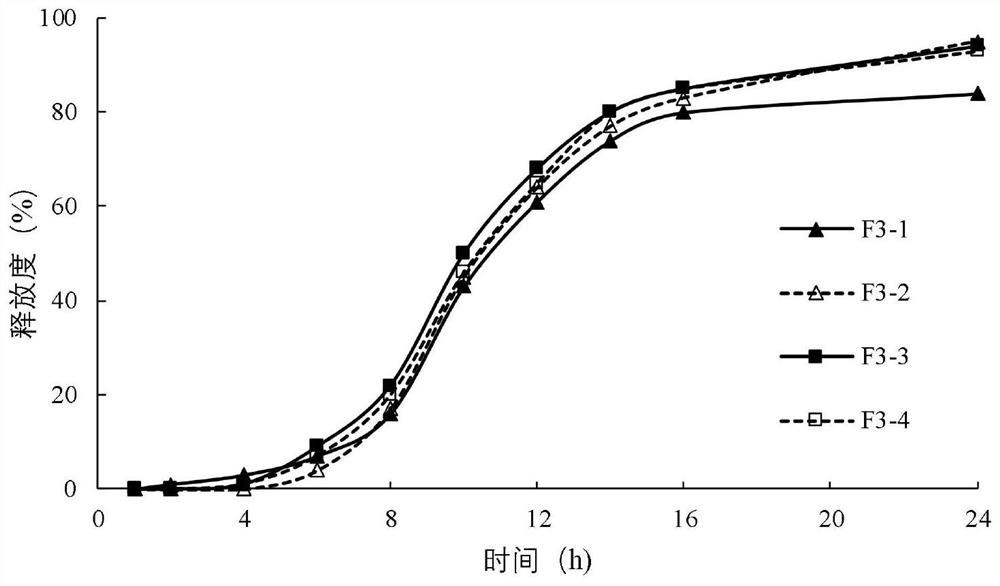
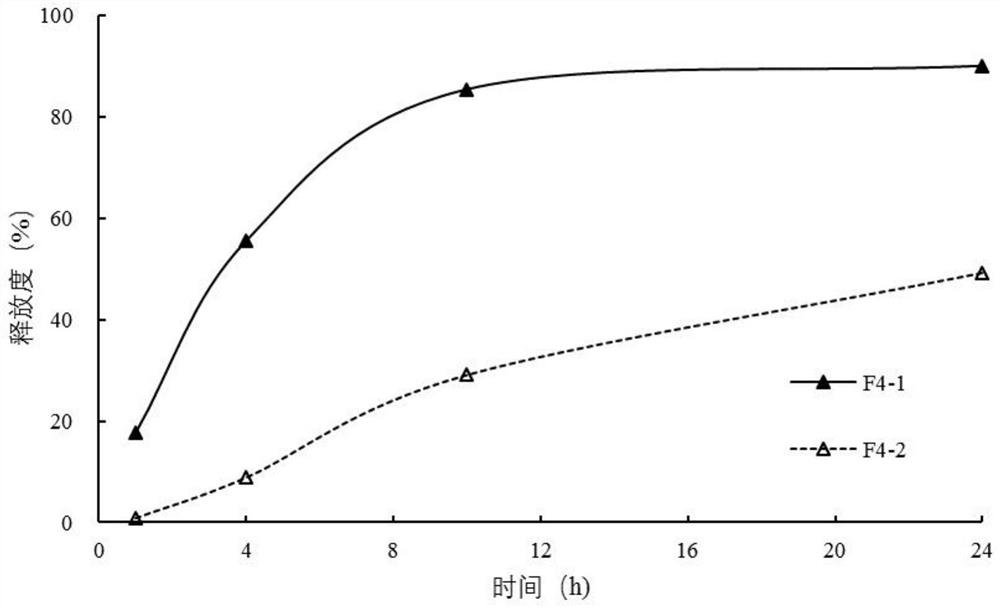
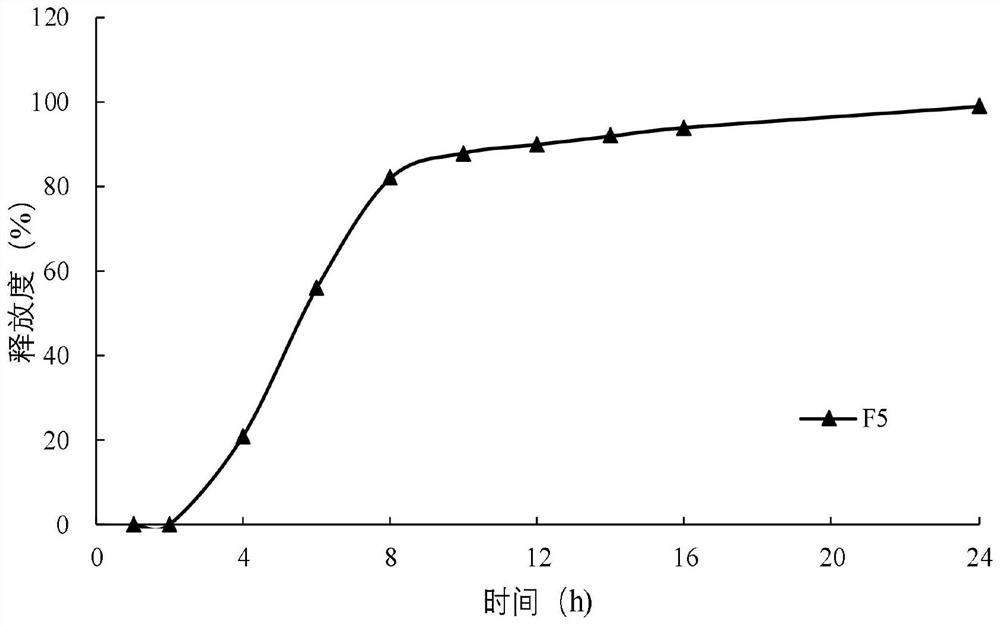
Diltiazem hydrochloride sustained-release capsule
A technology for diltiazem hydrochloride and capsules, applied in the field of medicine, can solve the problems of complex operation process, unqualified, and high requirements for pharmaceutical equipment, and achieve the effects of stable drug release, simple preparation process and controllable quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1, extrusion spheronization method prepares quick-release pellet part
[0070] 1. Prescription
[0071] components use F1(%w / w) diltiazem hydrochloride Main drug 71.8 Plasdone K-29 / 32 Adhesive 3.0 Microcrystalline Cellulose PH101 filler 13.0 Tween 80 D 1.5 fumaric acid Acidifier 10.7 water solvent Appropriate amount (volatile during drying) total / 100.0
[0072] 2. Preparation method
[0073] Weigh each component of the prescription amount, fully mix diltiazem hydrochloride, microcrystalline cellulose PH101, and fumaric acid, and use an aqueous solution containing Plasdone K-29 / 32 and Tween 80 to carry out wet granulation, and the obtained wet granules are Prepare pellets by extrusion and spheronization, then put the wet pellets into a fluidized bed to dry, and sieve the dried pellets to remove larger aggregates on the sieve and fine powder under the sieve to obtain the product.
Embodiment 2
[0074] Embodiment 2, fluidized bed medicine method prepares quick-release pellet part
[0075] 1. Prescription
[0076]
[0077] 2. Preparation method:
[0078] Weigh each component of the prescription amount, dissolve diltiazem hydrochloride, Plasdone K-29 / 32 or HPC (Klucel EXF) and Tween 80 in water to prepare a drug solution, and the drug solution passes through the spray gun system under the effect of atomizing air Coated on the surface of the fluidized sucrose core to form a drug layer. After the drug solution is sprayed, the fumaric acid solution is continuously sprayed into the fluidized bed, and a fumaric acid layer is coated on the surface of the drug layer. Sieve the dried pellets to remove larger aggregates on the sieve and fine powder under the sieve to obtain the product.
Embodiment 3
[0079] Embodiment 3, the coating method of the present invention prepares sustained-release pellet capsule
[0080] 1. Prescription
[0081]
[0082]
[0083] 2. Preparation method
[0084] The sustained-release coating layer suspension is coated on the surface of the fluidized drug-containing immediate-release pellets through a spray gun system under the action of atomized air to form a sustained-release coating layer. Sieve the dried sustained-release pellets to remove larger aggregates on the sieve and fine powder under the sieve. The obtained sustained-release pellets are mixed with talcum powder for lubrication, filled into capsules, and obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com