Method for enriching and purifying steroidal alkaloid in fritillaria medicinal material
A technology of steroidal alkaloids and alkaloids, which is applied in the field of enrichment and purification of steroidal alkaloids, can solve the problems of large solvent consumption, obstruction, low recovery rate, etc., achieve fast enrichment methods, overcome low solubility, high Variety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] Preparation of stationary phase FC8HL: add 10.3g of hydrated silica gel (silica gel water absorption rate 3%) to a 250mL flask, add 100mL of xylene, stir evenly, and then dropwise add 5.5g of 1H, 1H, 2H, 2H-perfluorooctyltrichlorosilane (moles: 11 mmol) and 4.1 g of 2-(4-chlorosulfonylphenyl)ethyltrichlorosilane (moles: 12 mmol) mixture, react at 30°C for 3 hours, filter, use dichloromethane and methanol successively , washed with methanol water, methanol, and tetrahydrofuran in a volume ratio of 1:1, and the obtained solid was dried in a drying oven at 80°C for 24 hours to obtain a stationary phase FC8HL, with the following structure:
[0033]
[0034] The content of fluorine-containing alkyl chains per g of silica gel is 1.1 mmol, and the polar group is 1.2 mmol; the stationary phase prepared above is used as a filler to be packed into an FC8HL chromatographic column and applied in the following examples;
[0035] The isosteroidal alkaloids described in the present...
Embodiment approach
[0039] 1. Reference substance preparation: respectively take the isosteroidal alkaloid reference substances fritillin A, fritillin B, fritillin and sambarine, and the steroidal saponin reference substances timosaponin BII, timosaponin AIII and Dioscorea saponin is appropriate amount, accurately weighed, and methanol is added respectively to make a stock solution containing 1000 μg of reference substance per 1 mL; each stock solution is diluted with methanol into a mixed standard solution with a concentration of 10 μg / mL of each component, and passed through a 0.22 μm organic membrane to remove impurities post analysis.
[0040] 2. Analysis conditions:
[0041] Instrument: Agilent 1290 UHPLC-QToF6540
[0042] Chromatographic column: FC8HL (Acchrom-Tech, 4.6×100mm, packing particle size 5μm)
[0043] Flow rate: 1.5mL / min (split)
[0044] Column temperature: 40℃
[0045] Injection volume: 4μL
[0046] Wavelength collection range: 190nm-400nm
[0047] Mass spectrometry param...
Embodiment 2
[0055] Method of implementation
[0056] 1. Sample preparation: The fritillary medicinal material was extracted with 15 times (medicine material g / mL solvent) volume concentration of 90% ethanol for 120 minutes, solid-liquid separation, and the extract was concentrated at 50 °C to 1 / 10 of the volume of the extract. , lyophilized total extract.
[0057] 2. Preparation of the test solution: take an appropriate amount of the total extract of Fritillaria fritillary, add an appropriate amount of methanol, and ultrasonically dissolve it to prepare a solution containing 200 mg of total extract per 1 mL.
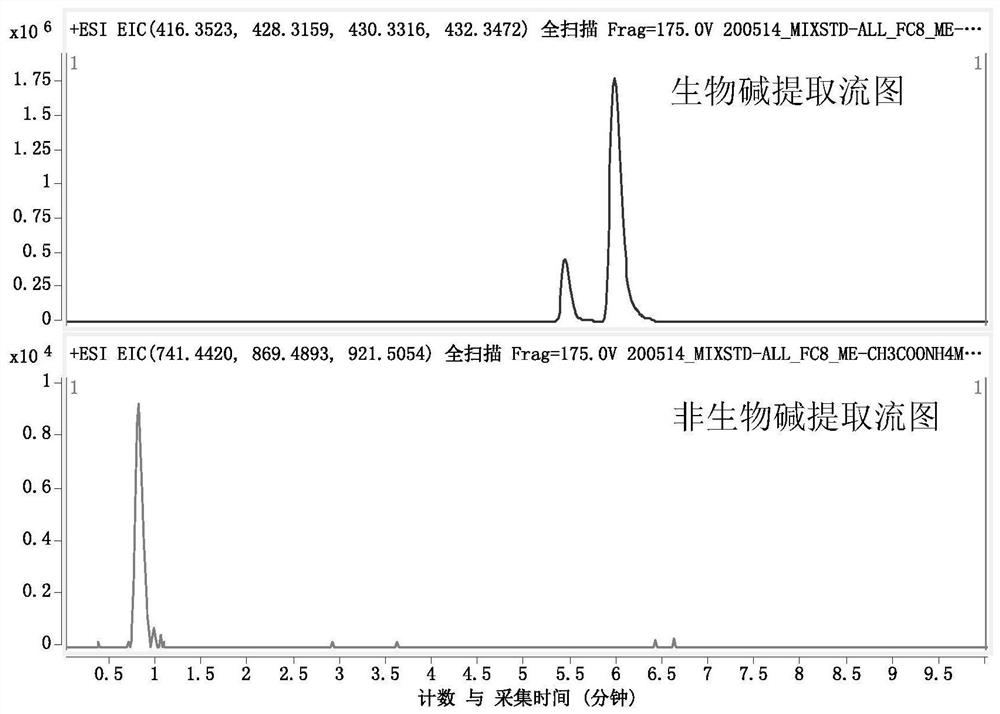
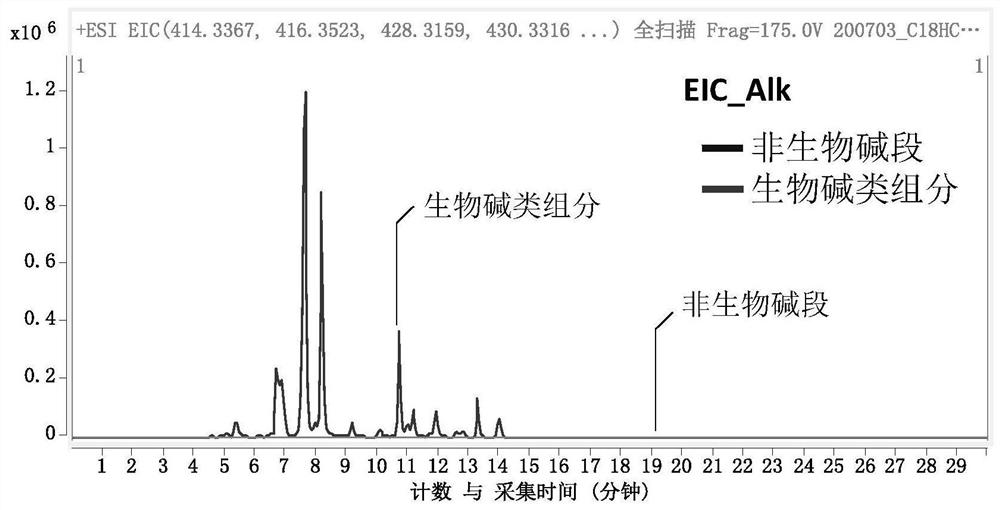
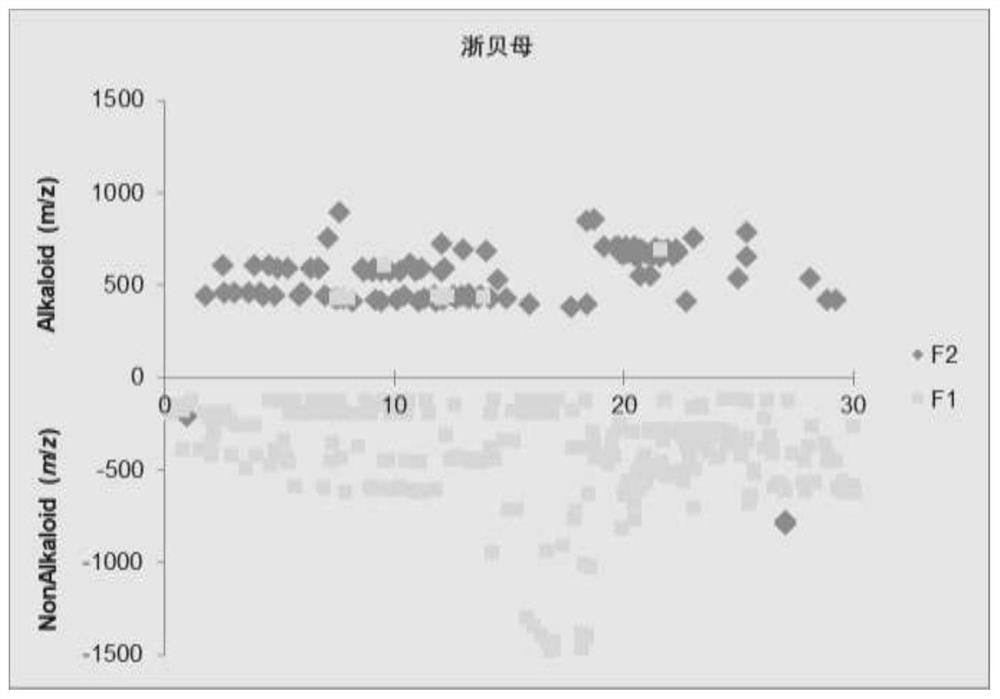
[0058] 3. Solid-phase extraction process: Take 20g FC8HL solid-phase extraction (SPE) column, activate and equilibrate with 3 times the column volume of methanol, and load the total extract with 1% of the sample (g / g); use 5 times the column volume The SPE column was rinsed with volume methanol to elute the non-alkaloid component (F1); the SPE column was rinsed with a 20 mM ammoniu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com