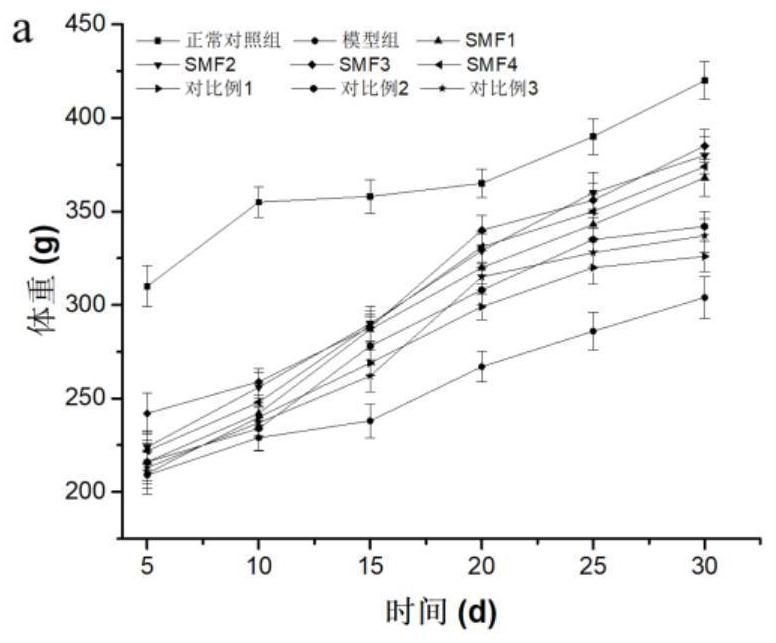
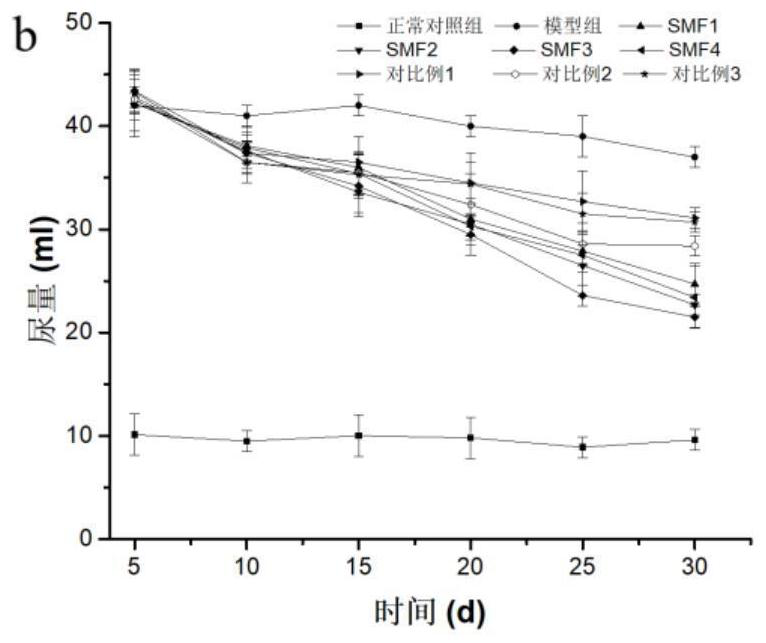
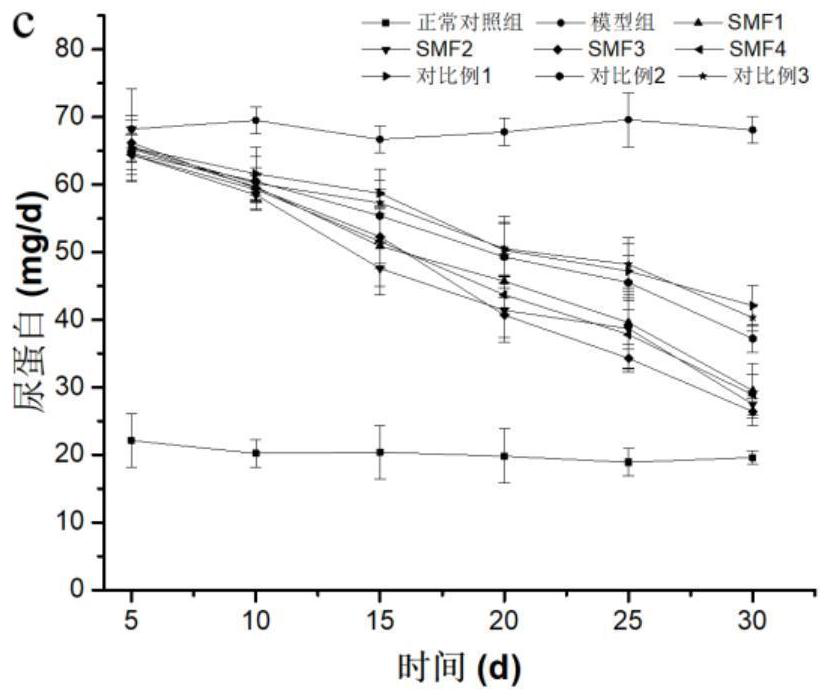
Medical nutrition powder for non-dialysis patients with nephropathy and preparation method of medical nutrition powder
A nutritional powder and patient technology, applied in the direction of food science, etc., can solve the problems of high prevalence of chronic kidney disease, high medical expenses, low awareness rate, etc., and achieve the effect of simple process steps, reduced losses, and controllable process parameters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0117] The application provides a preparation method of the above-mentioned medical nutritional powder for non-dialysis patients with renal disease, comprising the following steps:
[0118] Step (1): take the first premix, dissolve in water, and mix to obtain the first mixture;
[0119] Step (2): take by weighing the second premix, put into the homogenizer with the first mixture obtained in step (1) and perform homogenization to obtain the second mixture;
[0120] Step (3): Sterilize and spray dry the second mixture obtained in step (2) to obtain the third mixture;
[0121] Step (4): Weigh the third premix and add it to the third mixture, and stir evenly to obtain a medical nutritional powder for non-dialysis patients with renal disease.
[0122] In a preferred embodiment of the present application, the first premix includes whey protein isolate powder, whey protein concentrate powder, soy protein isolate, casein, maltodextrin, crystalline fructose, iso-oligomerization Malto...
Embodiment 1
[0143] Step (1) Preparation of the first mixed material: taking the mass of protein as 100 g, weigh the first premix, specifically 37.18 g of whey protein isolate powder, 25.64 g of whey protein concentrate powder, and 17.95 g of soybean protein isolate , casein 12.82g, maltodextrin 376.92g, crystalline fructose 79.49g, isomalt oligosaccharide 87.18g, fructooligosaccharide 25g, resistant dextrin 48.72g, inulin 61.54g, soybean fiber 38.46g, sodium citrate 0.179 g, potassium citrate 2.95g, copper sulfate 0.002g, magnesium chloride 1.38g, ferrous sulfate 0.04g, zinc sulfate 0.02g, manganese sulfate 0.0002g, calcium carbonate 2.56g, disodium hydrogen phosphate 1.15g, potassium iodide 0.00015g, Sodium selenate 0.00002g, vitamin C1.4g, vitamin B1 0.02g, vitamin B2 0.02g, vitamin B6 0.02g, vitamin B12 0.00003g, folic acid 0.006g, pantothenic acid 0.075g, biotin 0.0005g, carrageenan 3.85g, melon 15.38g of Er glue and 3.85g of vanilla essence, dissolved in water with a temperature of 5...
Embodiment 2
[0148] Step (1) Preparation of the first mixed material: taking the mass of protein as 100g, weigh the first premix, specifically 35.17g of whey protein isolate powder, 27.83g of whey protein concentrate powder, and 17.74g of soybean protein isolate , casein 13.15g, maltodextrin 254.43g, crystalline fructose 53.82g, isomalt oligosaccharide 61.47g, fructooligosaccharide 53g, resistant dextrin 53.82g, inulin 37g, soybean fiber 25.38g, sodium citrate 1.83g , potassium citrate 28.62g, copper sulfate 0.02g, magnesium chloride 11.76g, ferrous sulfate 0.4g, zinc sulfate 0.18g, manganese sulfate 0.0009g, calcium carbonate 24.46g, disodium hydrogen phosphate 11.12g, potassium iodide 0.00153g, selenite Sodium 0.0012g, Vitamin C1.8g, Vitamin B1 0.028g, Vitamin B2 0.028g, Vitamin B60.028g, Vitamin B12 0.00004g, Folic acid 0.007g, Pantothenic acid 0.095g, Biotin 0.001g, Carrageenan 3.67g, Guar 17.43g of glue and 3.06g of vanilla essence, dissolved in water with a temperature of 55°C and a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com