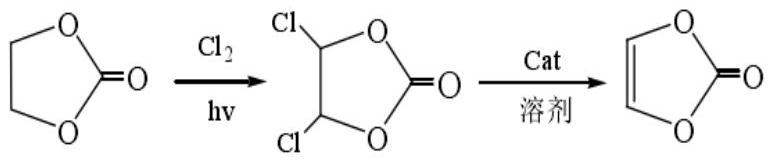
Vinylene carbonate production method and production system
A technology of vinylene carbonate and ethylene carbonate, which is applied in the field of production methods and production systems of vinylene carbonate, can solve the problems of large-scale economy of the process system, troublesome by-product treatment, low purity of crude products, etc., and achieve omission of solvent removal Eliminate steps, reduce energy consumption, and achieve high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
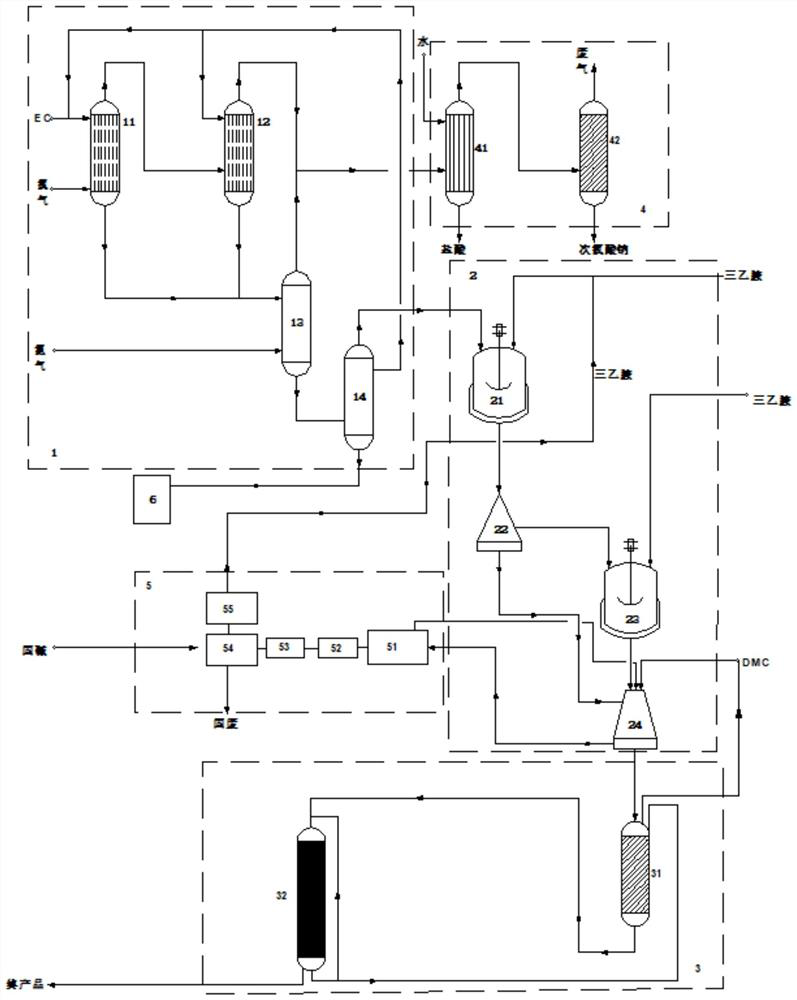
[0064] using as figure 1 The production system shown produces vinylene carbonate and includes a primary reaction unit 1 , a secondary reaction unit 2 , a purification unit 3 , a by-product recovery unit 4 and a triethylamine recovery unit 5 .
[0065] The primary reaction unit 1 includes a first photolysis reaction tower 11, a second photolysis reaction tower 12, a deacidification tower 13 and a rectification tower 14. The first photolysis reaction tower 11 and the second photolysis reaction tower 12 are used as chlorination towers. The reaction vessel is provided with an ultraviolet light assembly (not shown) and a temperature adjustment assembly (not shown), the ultraviolet light assembly is used to provide ultraviolet light conditions for the chlorination reaction, and the temperature adjustment assembly is used to adjust the chlorination reaction. temperature. The photolysis reaction tower is in the prior art and will not be repeated here.
[0066] The first photolysis r...
Embodiment 2
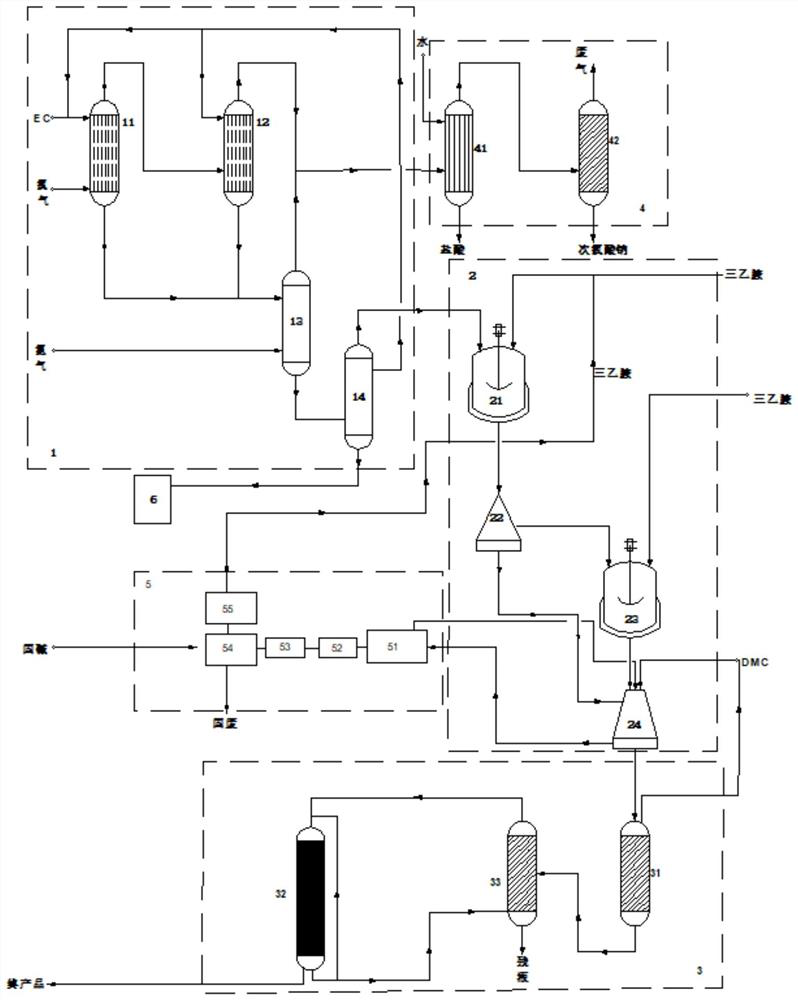
[0111] like figure 2 The production system shown, the difference between this example and Example 1 is: the temperature of the precipitation treatment is 40 ° C, the pressure is 10 kPa; the communication pipeline between the precipitation tower 31 and the falling film crystallizer 32 The distillation column 33 and the falling film crystallizer 32 are communicated with the lower part or bottom of the crude distillation column 33 through a circulating pump (not shown). Impurities such as polymerization inhibitor butyl-p-cresol (BHT) and polyethylene carbonate can be removed by distillation through the crude distillation column 33, thereby further improving the purity of the final product. The temperature of the crude distillation treatment is 55°C, and the pressure is 2kPa; the purity of the material after being processed by the crude distillation column 32 is 98%;
[0112] The molar ratio of the total amount of chlorine gas to the total amount of ethylene carbonate in the fir...
Embodiment 3
[0138] The difference between this example and Example 2 is that the molar ratio of the total amount of chlorine gas to the total amount of ethylene carbonate in the first photolysis reaction tower 11 and the second photolysis reaction tower 12 is 0.5:2.5, and the triethylamine The molar ratio of the total amount to the total amount of high-purity chlorinated ethylene carbonate (CEC) is 1:1.2, and the molar ratio of the total amount of vinylene carbonate to the total amount of high-purity chlorinated ethylene carbonate (CEC) is 1:1.2, The mass ratio of the total amount of butyl-p-cresol (BHT) to the total amount of high-purity chlorinated ethylene carbonate (CEC) is 0.3:100, the temperature of the chlorination reaction is 64°C, the time of the chlorination reaction is 1.5h, and the chlorine The content of dichloroethylene carbonate in the substitution reaction liquid is 0.71wt%; the temperature of the dechlorination reaction is 64°C, and the time of the dechlorination reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com