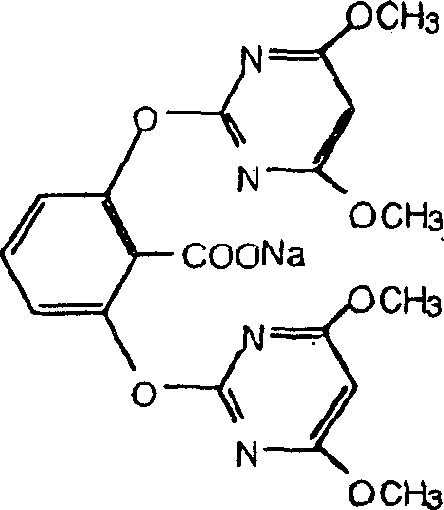
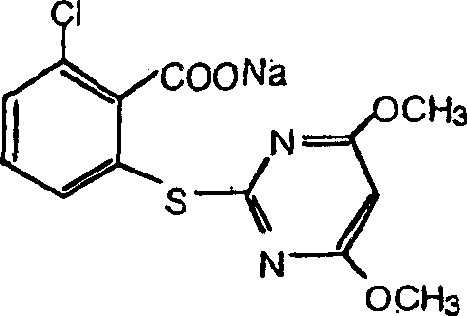
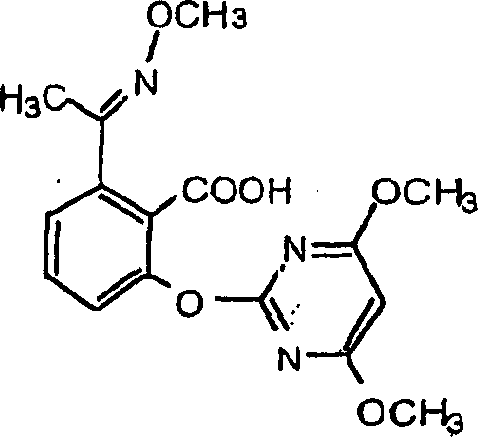
Genes encoding acetolactate synthase
An acetolactate synthase, gene technology, applied in genetic engineering, plant genetic improvement, lyase and other directions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] [Example 1] Preparation of rice (Kinmaze) cultured cells resistant to PC herbicides
[0097] Rice husks were removed from rice seeds (variety: Kinmaze, scientific name: Oryza sativa var. Kinmaze). Seeds were soaked in 70% ethanol for 5 minutes and then in 5% antiformin for 20 minutes, followed by several washes with sterile distilled water. The seeds were then cultured statically in media containing the compositions shown in Table 3.
[0098] Inorganic salts (mixed brine for Murashige-Skoog medium)
1 pack
Thiamine Hydrochloride (0.1 g / L)
1 ml
Niacin (0.5 g / L)
1 ml
Pyridoxine hydrochloride (0.5 g / L)
1 ml
Glycine (2 g / L)
1 ml
Inositol (50 g / L)
2ml
2,4-D (200ppm)
10ml
30 grams
Deacetylated gellan gum
3 grams
Prepare the medium to 1000 ml with distilled water and adjust the pH to 5.7
[0099] In the above medium composition, 2,4-D is a synthet...
Embodiment 2
[0107] [Example 2] The partially purified ALS protein from the resistant mutant is sensitive to herbicides
[0108] In this embodiment, the mutant ALS protein is partially purified from the resistant mutants obtained in Example 1 (except the Rb line, Sr line and Vg line, Ga line), and then the mutant ALS protein obtained is measured for herbicide activity. sensitivity. The mutant ALS protein was partially purified as follows.
[0109] First, using the composition shown in Table 3 but excluding gellan gum, 200 g or more of resistant mutants were prepared by liquid culture (without adding bispyribac-sodium). Then, use a Hiscotron in buffer solution-1 [100 mM potassium phosphate buffer (pH 7.5), which contains 20% (v / v) glycerol, 0.5 mM thiamine pyrophosphate (TPP) , 10 μM flavin adenine dinucleotide (FAD), 0.5 mM magnesium chloride and one-tenth tissue volume of polyvinylpolypyrrolidone] about 150 g of resistant mutants were homogenized. The homogenate was filtered through ny...
Embodiment 3
[0123] [Example 3] Cloning of wild-type and mutant ALS genes
[0124] In this example, the gene encoding the wild-type ALS protein (wild-type ALS gene) was cloned from the wild-type, and the gene encoding the mutant ALS protein (mutant ALS gene) was cloned from the resistant mutant.
[0125] Probes for cloning the wild-type ALS gene and the mutant ALS gene were prepared by the methods described below. Partial cDNA obtained from rice (Nippon-bare) showed high homology with maize ALS gene, and it was used as a probe in this example.
[0126] (1) Determination of the nucleotide sequence of a partial cDNA from rice (Nippon-bare) showing high homology with the corn ALS gene
[0127]As part of the rice genome project implemented by the Institute of Technology Innovation of Agriculture, Forestry and Fishery and the National Institute of Agricultural Biological Sciences, the nucleotide sequence of part of the cDNA of rice (Nippon-bare) has been determined, and the partial nucleus of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com