Cavity-transferring material and its preparation method
A technology of hole transport materials and compounds, applied in the field of hole transport materials, can solve problems such as low thermal stability and limited commercial application of materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
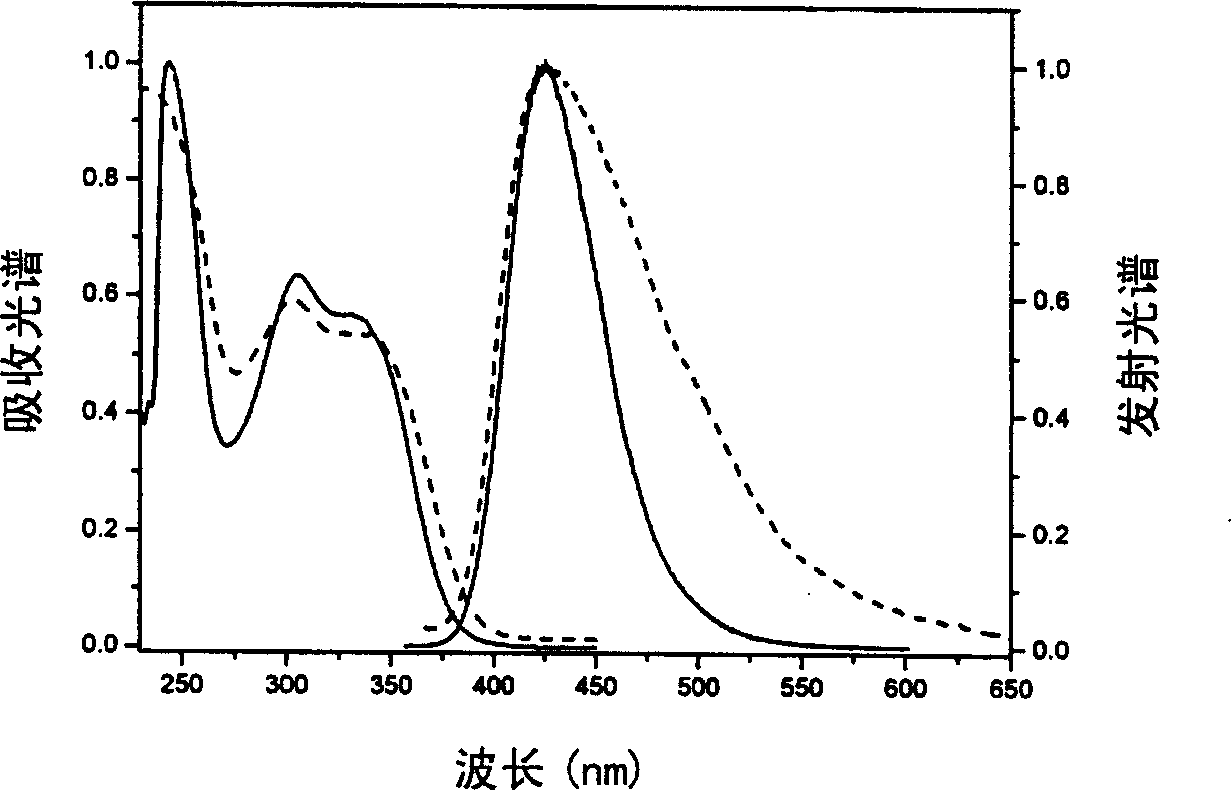
Image
Examples
Embodiment 1
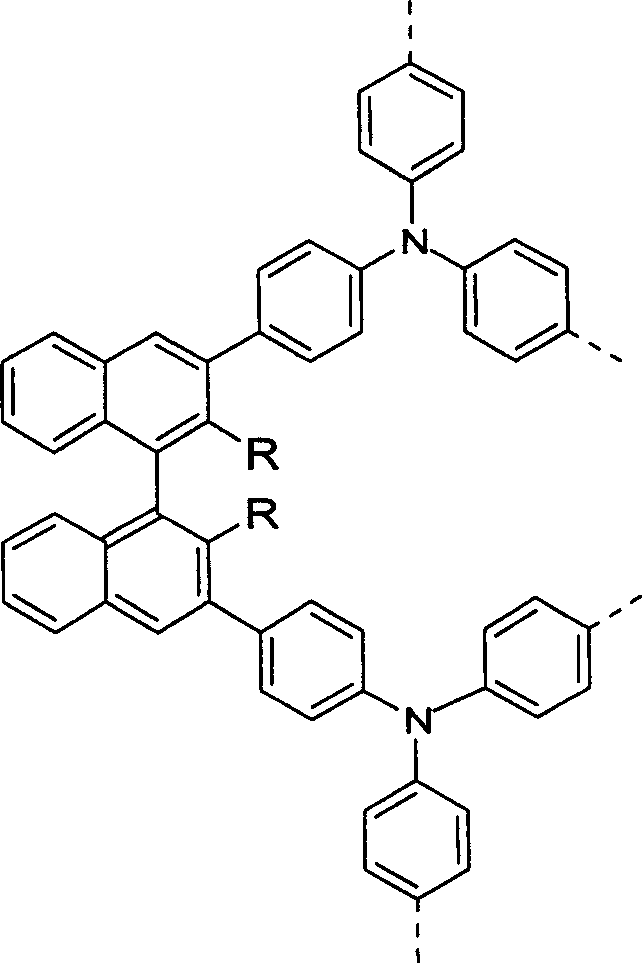
[0027] The reaction formula for preparing bis(N,N'-diphenyl)-2,2'-dimethoxy-1,1'-binaphthyl-3,3'-diamine (TPA-BN-TPA) compound as follows:
[0028]
[0029] Wherein, the central part of the molecule is 2,2'-dimethoxy-1,1'-binaphthyl, and the 3,3' position of the binaphthyl chromophore is triphenylamine.
[0030] The specific preparation method is as follows:
[0031] First prepare compound 1, the synthesis process is as follows:
[0032] Add 0.648g (2.0mmol) of 4-bromophenyldiphenylamine and 0.402g (1.0mmol) of binaphthyl diboronic acid into a 50mL two-necked flask, repeat the vacuum-filling process three times, and add 15mLTHF to dissolve them. Stir, add Pd(PPh 3 ) 4 40 mg, K 2 CO 3 15 mL of 1M aqueous solution. Heat to reflux for 12 hours, extract the organic phase with dichloromethane, and combine the organic phases. Wash with 1M hydrochloric acid and saturated brine, and dry over anhydrous magnesium sulfate. Filtrate, remove the solvent by rotary evaporation, ...
Embodiment 2
[0038] 9-hexyl-3-(1-(2-(9-hexyl-9H-carbazol-3-yl)-3-methoxynaphthalen-4-yl)-2-methoxynaphthalen-3-yl)-9H-carbazole(Cz- Synthesis of BN-Cz)
[0039]
[0040] Add 0.66 g (2.0 mmol) of 3-bromo-9-hexylcarbazole (3-iodo-9-hexylcarbazole or other monohalogenated arylamine compounds) to a 50 mL two-necked flask, 0.402 binaphthyl diboronic acid g (1.0mmol) (the methoxy group of the diboronic acid here can also be other symmetrically or asymmetrically substituted alkyl or aryl groups), repeat the process of evacuating and argon filling three times, and add 15mL dioxane (also available Ethylene glycol dimethyl ether, tetrahydrofuran, toluene, and dichloromethane instead) were dissolved. Stirring, adding Pd under nitrogen protection 2 (dba) 3 40mg (also available Pd(OAc) 2 , or Pd(PPh 3 ) 2Cl 2 / PPh 3 (1:1), Pd(PPh 3 ) 2 Cl 2 / POT(1:1), Pd(PPh 3 ) 2 Cl 2 / P(t-Bu) 3 ) (1:1), tetra-n-butylammonium bromide 0.65g (2mmol) KOH (also can be NaOH or Ba(OH) 2 ) 15 ml of 1M aque...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com