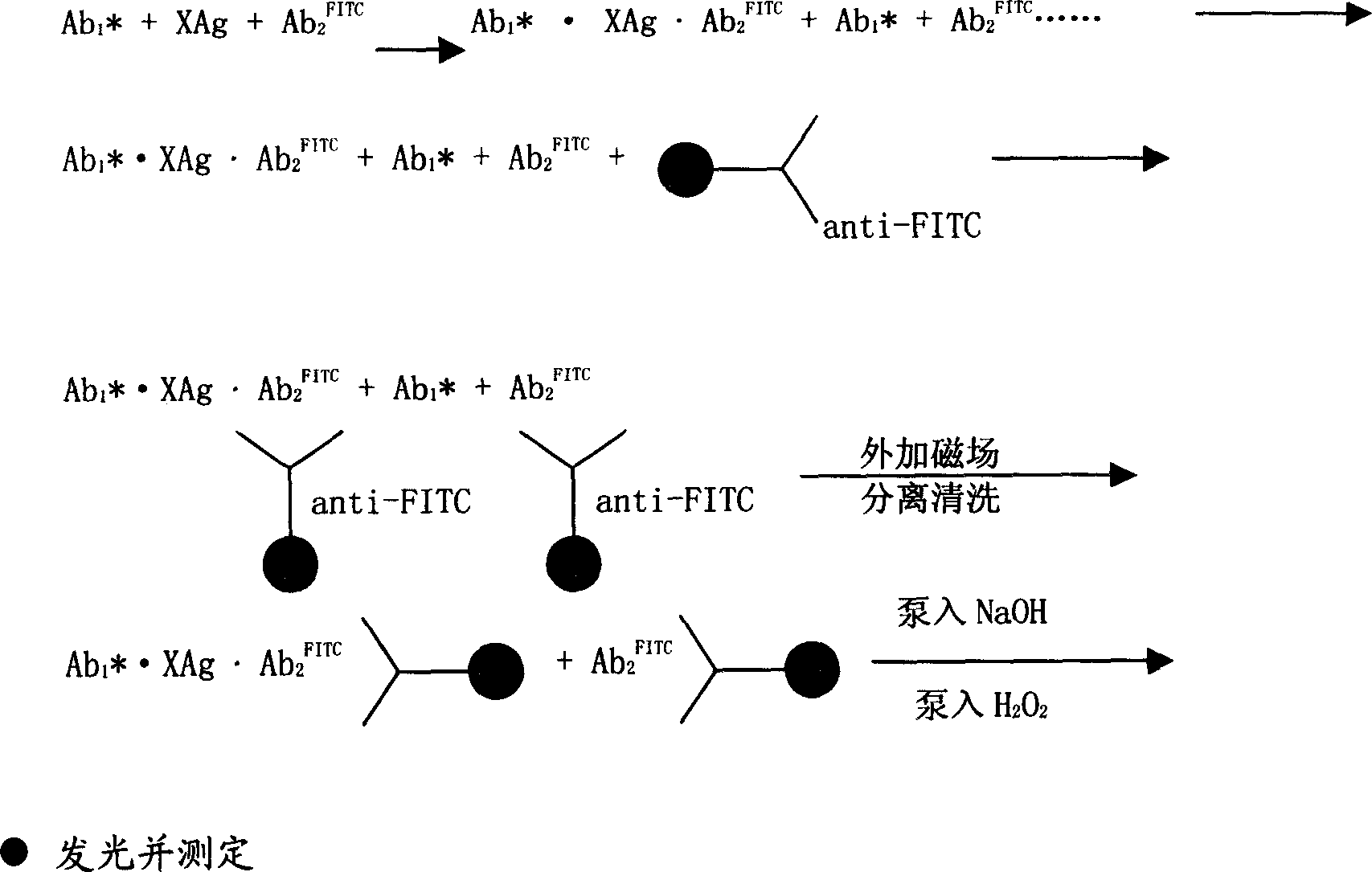
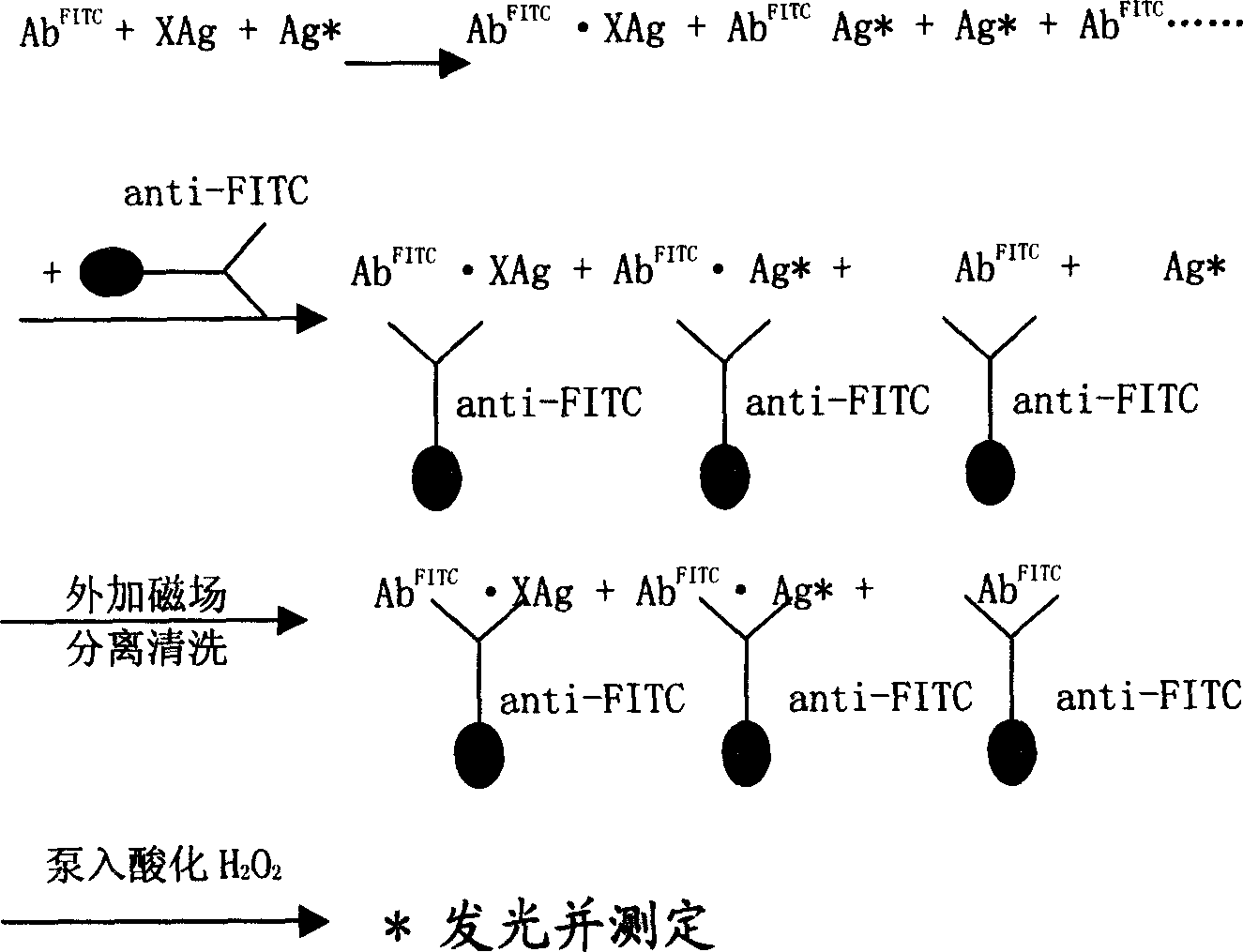
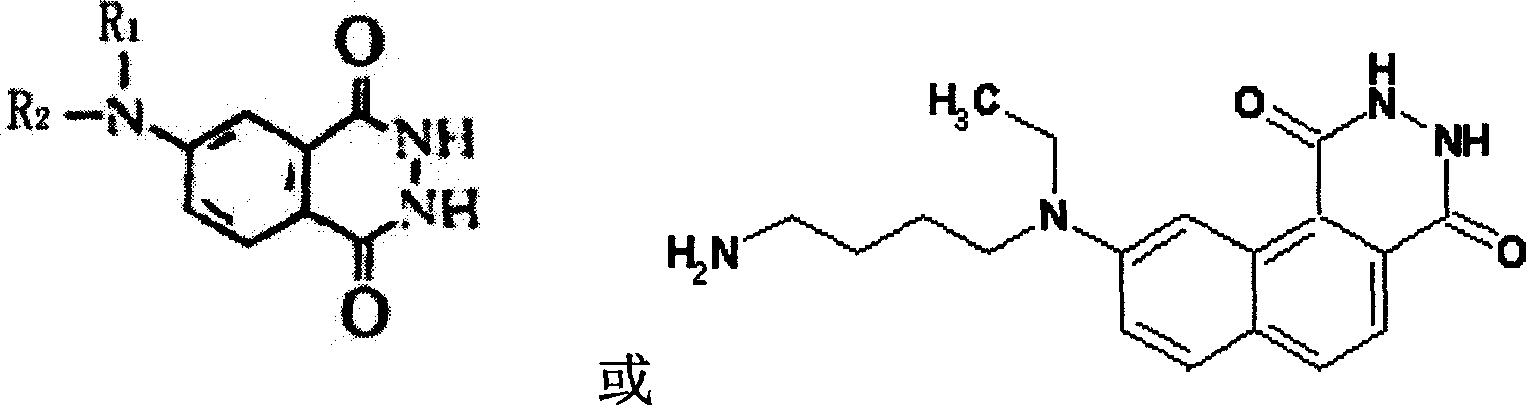
Magnetic separating direct chemical illuminating reagent and testing method using the same reagent
A chemiluminescent reagent and magnetic separation technology, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve the problems of unstable reagents, hindered by trace active substances, insufficient sensitivity, etc., so as to avoid the easy decline of enzyme activity and simplify the Immunological design to overcome the effect of easy hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Preparation of monoclonal antibody labeled with isoluminol derivatives:
[0033]Take three test tubes of 3.2mmol ABEI, dissolve them in 0.2ml of secondary water, 3.5mmol of CSCl 2 Dissolve in 0.3ml DMF, mix the two evenly, react at room temperature for 2 hours, take 10mg anti-CEA-α, anti-AFP-α and 10mg anti-PSA-α monoclonal antibody respectively, adjust the volume with pH9.5 carbonate buffer to 1ml, add the above-mentioned activated ABEI solution, mix well, react at room temperature for 20h, and purify by G-25 gel column;
[0034] 2. FITC-labeled CEA, AFP, PSA monoclonal antibody
[0035] Take 10mg of anti-CEA-β, AFP-β and PSA-β monoclonal antibodies, slowly adjust the volume to 1ml with pH9.5 carbon, add FITC100ug, react at room temperature for 20 hours, and purify through G-25 gel column;
[0036] 3. Preparation of goat anti-FITC polyclonal antibody coated on the surface of nano-magnetic microbeads:
[0037] The preparation of nano-magnetic micro-beads is prepar...
Embodiment 2
[0046] The preparation of monoclonal antibody of isoluminol derivatives and the preparation method of goat anti-FITC polyclonal antibody coated on the surface of nano-magnetic microbeads are the same as in Example 1. The luminescent marker used in this example is AHEI, and the surface of the magnetic beads is The group containing -COOH, its content is 0.3eq / g;
[0047] Take TSH, T3, T4, FT3 and FT4 standard products, 20 μl each of serum samples, add 40 μl each of AHEI and FITC monoclonal antibodies of the luminescent marker monoclonal antibody, mix well and incubate at 37°C, in a water bath 15 minutes; after the immune reaction occurs, add 40 μl of immune nano-magnetic beads separation reagent coated with goat anti-FITC polyclonal antibody on the surface, mix well, and bathe in water for 5 minutes. Under the action of an external magnetic field, separate on the upper separator for 4 minutes, pour Supernatant; add 400 μl of washing solution, mix well, separate for 4 minutes, po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com