Liver target high molecule magnetic resonance image-forming contrast medium and its synthesizing method and application
A technology of magnetic resonance imaging and synthesis method, which is applied in pharmaceutical formulations, genetic material components, preparations for in vivo experiments, etc. The effect of increasing the susceptibility and prolonging the cycle time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
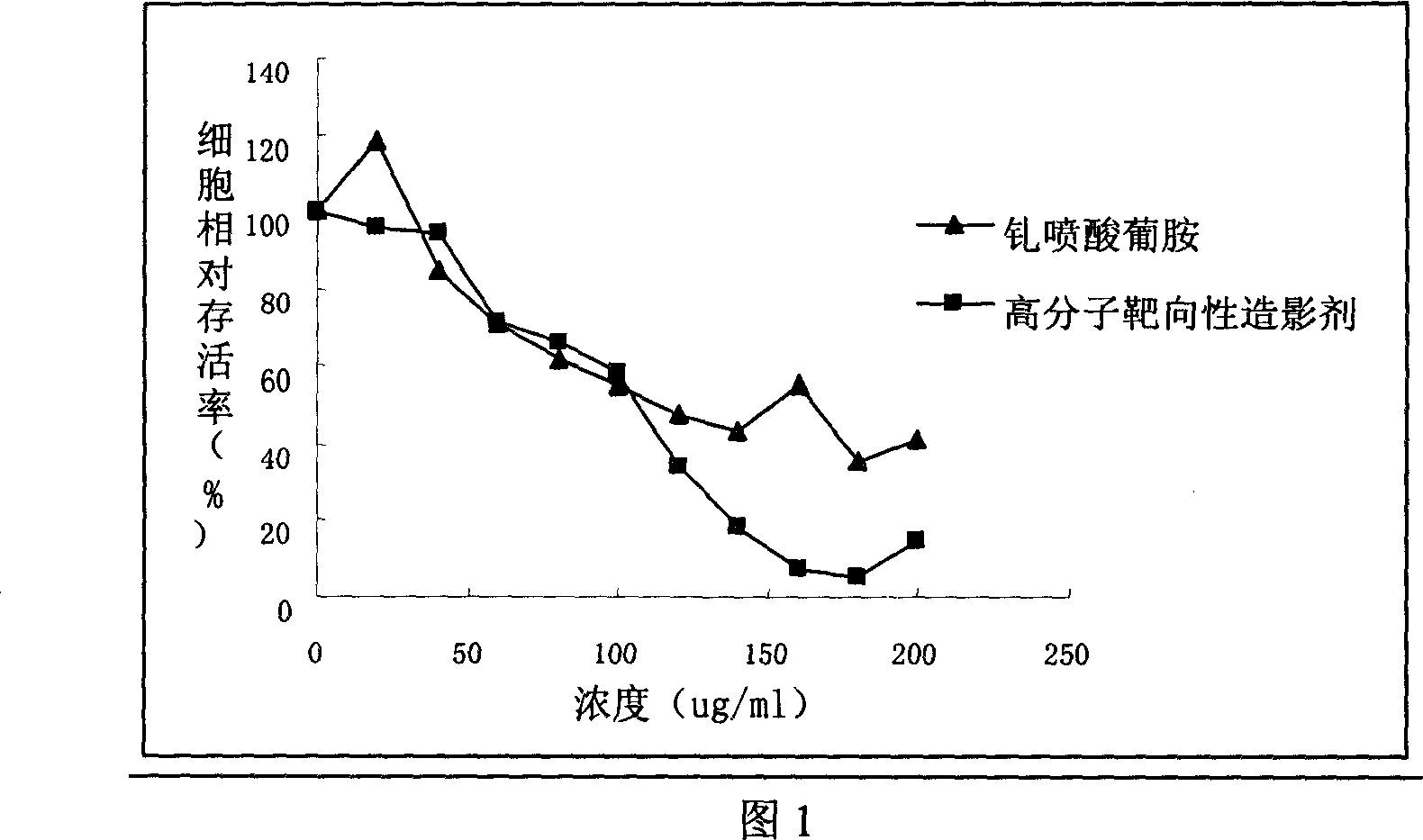
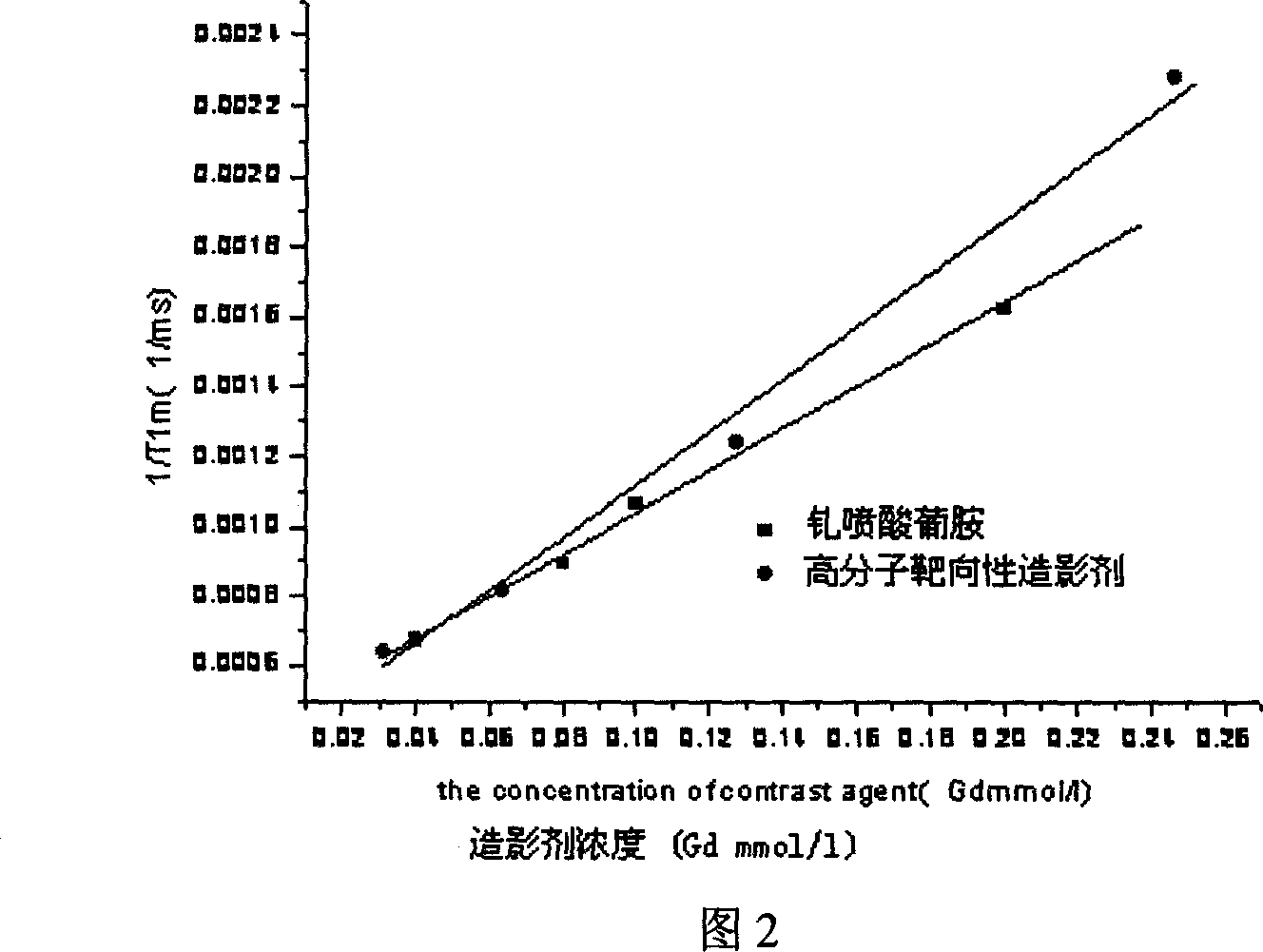
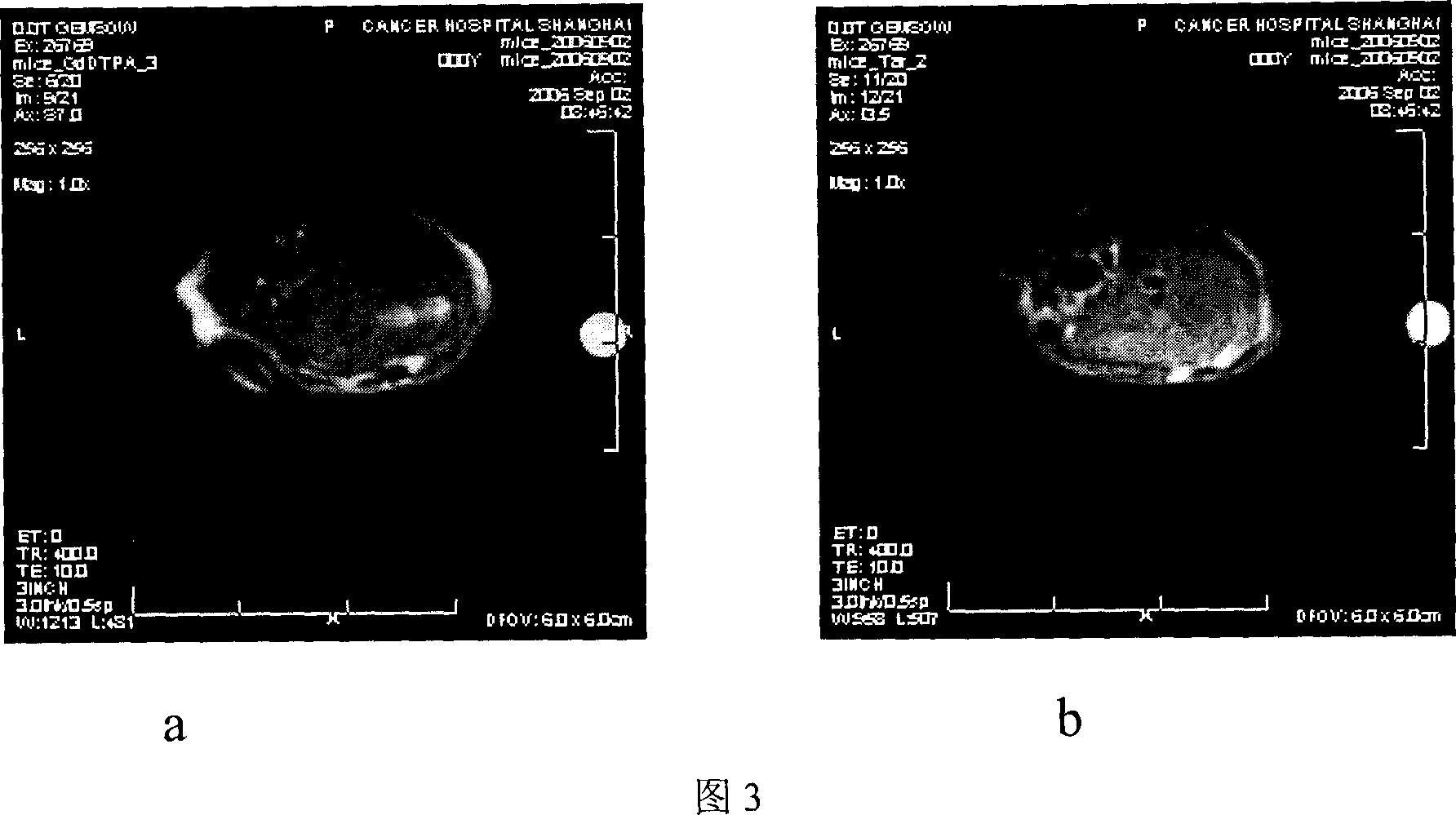
Image
Examples
Embodiment 1
[0051] Example 1α, the synthesis of β-poly[(2-aminoethyl)-L-asparagine] (PI)
[0052] Add 25 grams (0.19mol) of L-aspartic acid powder and 12.5 grams of 85% phosphoric acid in a 500ml round bottom flask, mix well, and react under reduced pressure at 185°C on a rotary evaporator for 3 hours until no more water vapor is produced. until. Add 100mL of DMF and stir to dissolve the solid matter, then slowly add the obtained solution dropwise into a beaker filled with 500mL of water under stirring, to obtain a white precipitate, let it stand, pour off the supernatant, filter, and wash the solid with water until neutral. Vacuum drying at 60° C. for 24 hours yielded 17 g of powdery solid with a yield of 93%.
[0053] Dissolve the prepolymer in DMF, add N,N-dicyclohexylcarbodiimide (DCC) and condense at room temperature for 24 hours, then filter and concentrate the filtrate. The product is precipitated with distilled water, collected and dried.
[0054] Dissolve 2.5 g (26 mmol) of the...
Embodiment 2
[0055] Coupling reaction (PII) of embodiment 2 lactobionic acid and polymer PI
[0056] Weigh 2 g of lactobionic acid (5.58 mmol) and dissolve it in 40 mL of phosphate buffer solution with pH=5.7, and cool in an ice bath. Add 20mL phosphate buffer solution of 2.6g (22.3mmol) N-hydroxysuccinimide and 4.3g (22.3mmol) EDC.HCl solid, stir in ice bath for 20 minutes, add polymer PII2.9g (18.6mmol), ice After bath reaction for 1 hour, the reaction was continued at room temperature for 36 hours. After 72 hours of dialysis of the reactant mixed solution bag, the solution was concentrated under reduced pressure to dryness to obtain 4.4 g of pale yellow solid PII, with a yield of 91%.
Embodiment 3
[0057] The synthesis of embodiment 3 macromolecular ligand (PIII)
[0058] (a) Synthesis of diethylenetriaminepentaacetic acid diactive ester
[0059] Weigh 8.6g of diethylenetriaminepentaacetic anhydride (DTPAA), 6.69g of N-hydroxysuccinimide and 100mg of 4-dimethylaminopyridine (DMAP) in 100ml of DMF and stir at room temperature for 24 hours. The product was precipitated with a 1:1 ethanol-ether mixture, washed with methanol, soaked in ether overnight, and dried to obtain 10.7 g of a white powdery solid with a yield of 76%.
[0060] (b) Synthesis of diethylenetriaminepentaacetic acid monoactive ester (Tyr-DTPA-OSu) containing tyrosine residues
[0061] Weigh 6.3541g (27.4mmol) methyl tyrosine hydrochloride and dissolve it in 30mlDMF, add 4ml triethylamine, stir and react for 20 minutes, filter out the white solid produced, transfer the filtrate to the dropping funnel, and slowly add it dropwise to In 40ml of DMF solution of diethylenetriaminepentaacetic acid bisactive este...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com