Cell fractions containing cells capable of differentiating into neural cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Bone Marrow Cells and Schwann Cells
(1) Bone Marrow Mononuclear Cells
[0045] Mouse bone marrow cells (10 μl) were obtained from the femur of adult LacZ (a structural gene of β-galactosidase) transgenic mice (The Jackson Laboratory, Maine, USA). The collected sample was diluted in L-15 medium (2 ml) containing 3 ml Ficoll, and centrifuged at 2,000 rpm for 15 minutes. Cells were collected from the mononuclear cell fraction, and suspended in 2 ml serum-free medium (NPMM: Neural Progenitor cell Maintenance Medium). Following centrifugation (2,000 rpm, 15 min), the supernatant was removed, and precipitated cells were collected and re-suspended in NPMM.
(2) Schwann Cells
[0046] Primary Schwann cell cultures were established from the sciatic nerve of neonatal mouse (P1-3) according to the method of Honmou et al. (J. Neurosci., 16(10): 3199-3208, 1996). Specifically, cells were released from sciatic nerve by enzymatic and mechanical treatment. 8×105 cells per plate were pla...
example 2
Experimental Animal Preparation and Transplantation
(1) Preparation of Demyelinated Rat Model
[0047] Experiments were performed on 12 week old Wistar rats. A localized demyelinated lesion was created in the dorsal columns using X-ray irradiation and ethidium bromide injection (EB-X treatment). Specifically, rats were anesthetized with ketamine (75 mg / kg) and xylazine (10 mg / kg) i.p., and a surface dose of 40 Grays of X-ray was irradiated using Softex M-150 WZ radiotherapy machine (100 kV, 1.15 mA, SSD 20 cm, dose rate 200 cGy / min) on the spinal cord caudal to the tenth thoracic spine level (T-10) through a 2×4 cm opening in a lead shield (4 mm thick). Three days after X-ray irradiation, rats were anesthetized as above, and aseptic laminectomy of the eleventh thoracic spine (T-11) was conducted. A demyelinating lesion was generated by the direct injection of ethidium bromide (EB) into the dorsal columns via a glass micropipette whose end was drawn. 0.5 μl saline containing 0.3 mg / ml...
example 3
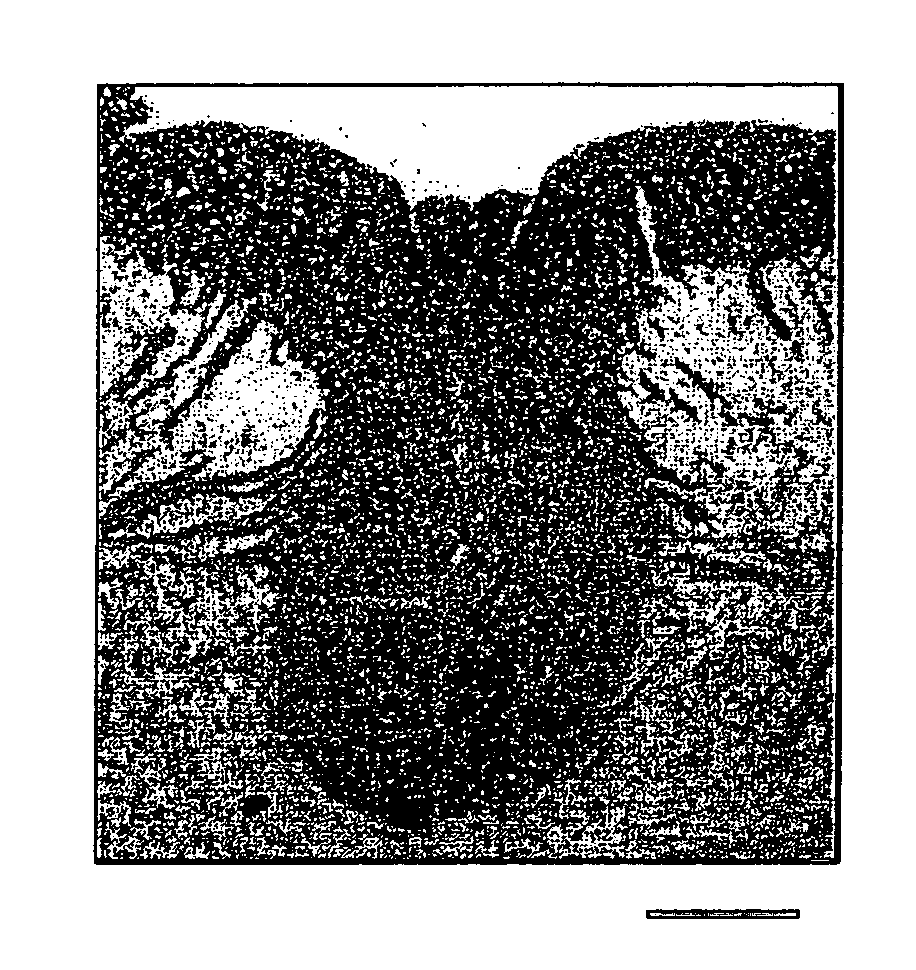
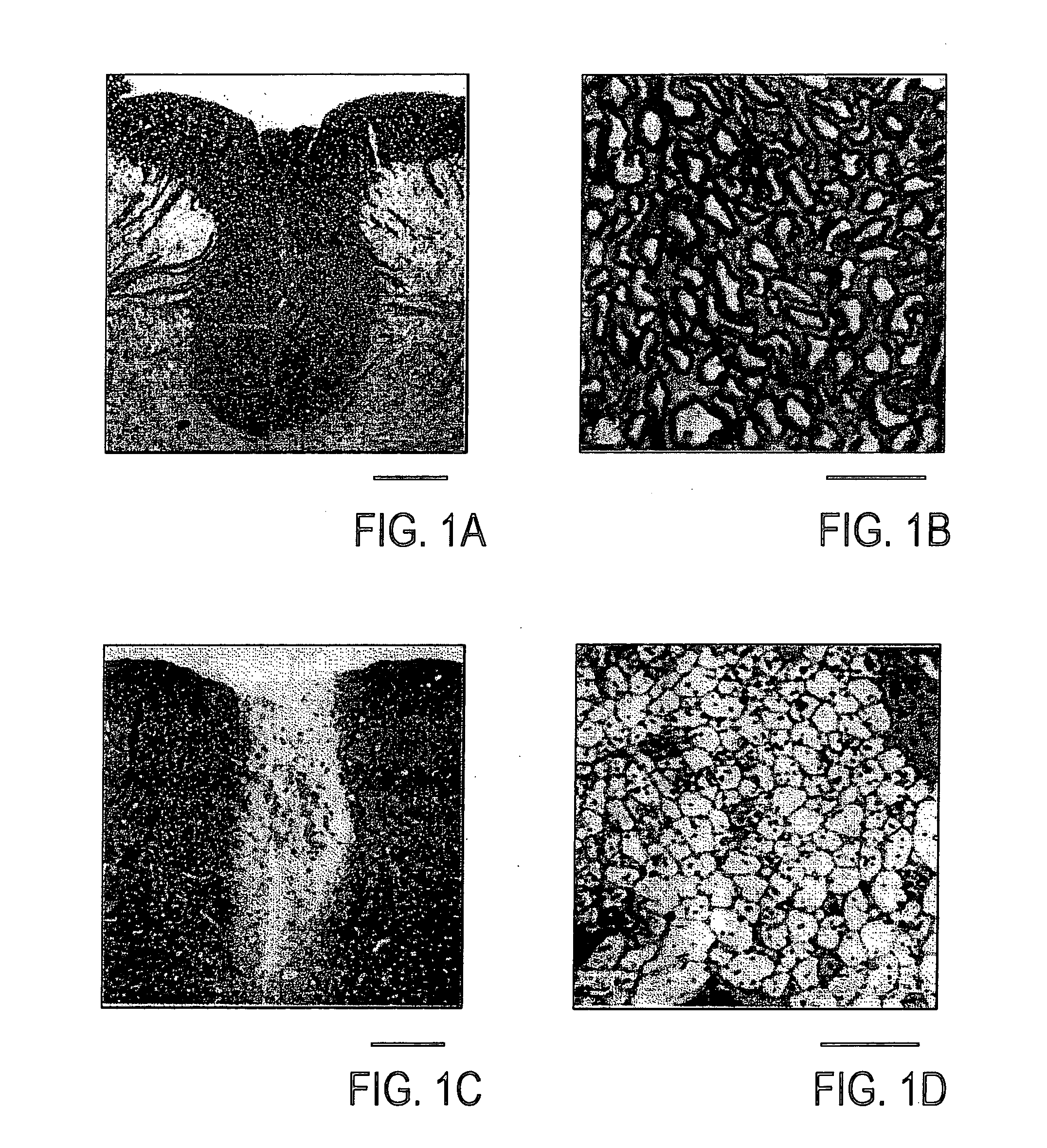
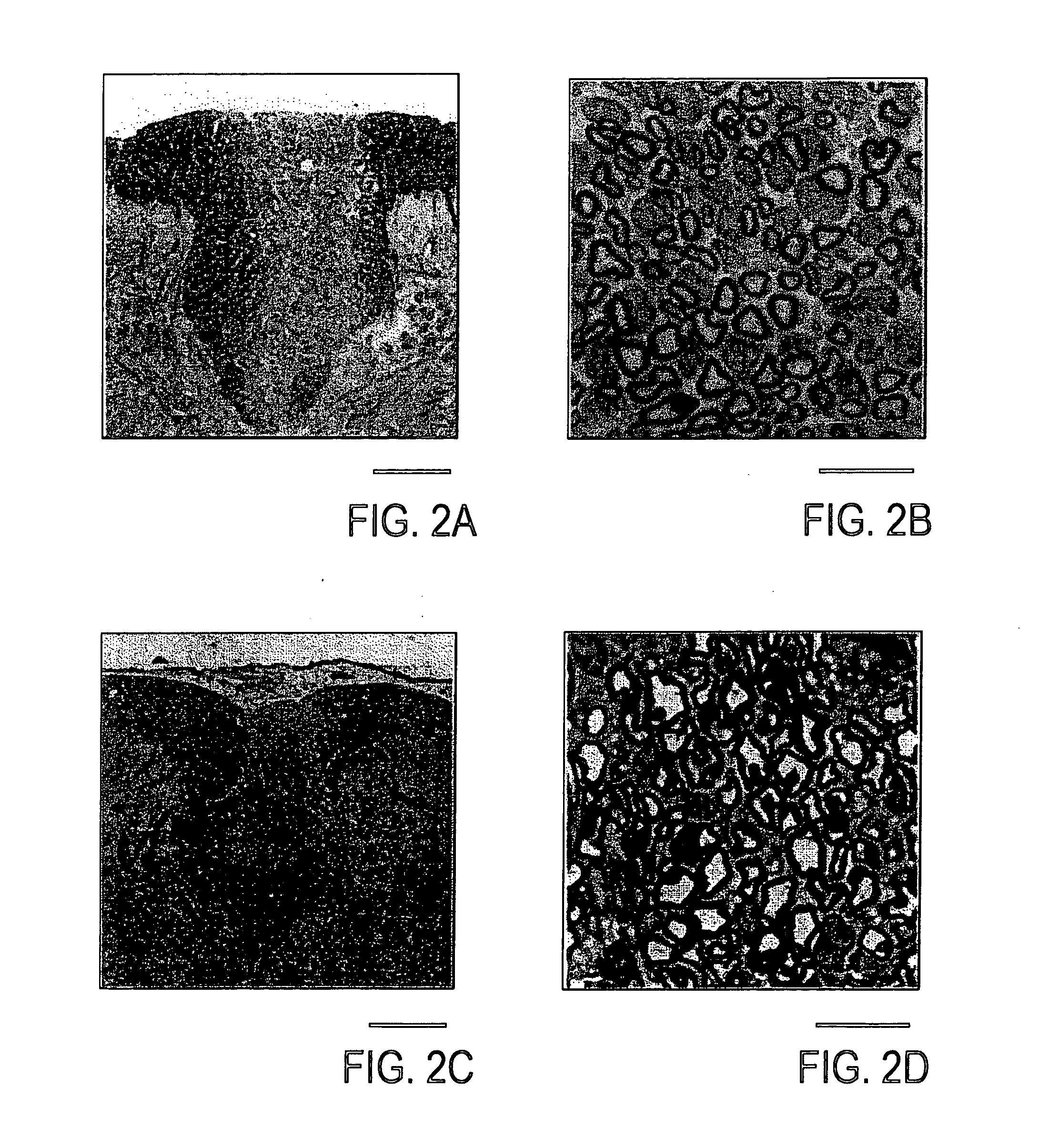
Histological Examination
[0049] Rats were deeply anesthetized by the administration of sodium pentobarbital (60 mg / kg, i.p.), and perfused through the heart cannula, first, with phosphate-buffer solution (PBS) and then with a fixative containing 2% glutaraldehyde and 2% paraformaldehyde in 0.14 M Sorensen's phosphate buffer, pH 7.4. Following in situ fixation for 10 minutes, the spinal cord was carefully excised, cut into 1 mm segments and kept in fresh fixative. The tissue was washed several times in Sorensen's phosphate buffer, post-fixed in 1% OsO4 for 2 hours at 25° C., dehydrated by elevating the concentration of the ethanol solution, passed through propylene oxide and embedded in EPON. Then, the tissue was cut into sections (1 μm), counterstained with 0.5% methylene blue and 0.5% azure II in 0.5% borax, and examined under light microscope (Zeiss: Axioskop FS). Ultrathin sections were counterstained with uranyl and lead salts, and examined with JEOL JEM1200EX electron microscop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com