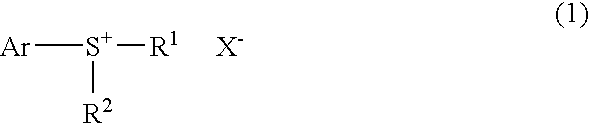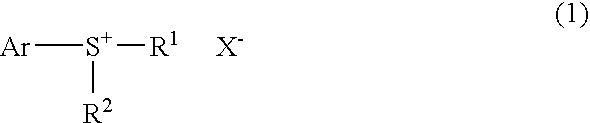Process for production of monosulfonium salts, cationic polymerization initiators, curable compositions, and products of curing
a monosulfonium salt and cationic polymerization technology, applied in the field of monosulfonium salt production, can solve the problems of reducing lowering the yield of sulfonium salts by several percent to about 10%, etc., and achieves excellent solubility in organic solvents, high purity and yield. , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0081] 4.28 g (23.3 mmol) of potassium hexafluorophosphate (KPF6), 10 ml of acetonitrile, 3.61 g (19.4 mmol) of diphenyl sulfide, 4.05 g (20.0 mmol) of diphenyl sulfoxide, and 5.94 g (58.2 mmol) of acetic anhydride were introduced into a 100 ml reaction vessel and uniformly mixed, and then 2.28 g (23.3 mmol) of concentrated sulfuric acid was added dropwise at room temperature over a period of 60 minutes. Heat was generated during this course to increase the temperature, but the system was cooled so that the temperature did not exceed 40° C. After 1 hour of stirring at 40° C., the system was cooled to room temperature, and 20 ml of water were added and stirred for 10 minutes, whereupon an oily substance became separated. 20 ml of ethyl acetate were added to this to dissolve the oily substance, and the organic layer was taken. This organic layer was washed with 10 ml of 20% caustic soda and washed three times with 10 ml of water, and then the acetonitrile and ethyl acetate were distil...
example 2
[0083] 3.91 g (23.3 mmol) of sodium hexafluorophosphate (NaPF6), 8 ml of acetonitrile, 3.61 g (19.4 mmol) of diphenyl sulfide, 4.05 g (20.0 mmol) of diphenyl sulfoxide, and 5.94 g (58.1 mmol) of acetic anhydride were introduced into a 100 ml reaction vessel and uniformly mixed, and then 2.28 g (23.3 mmol) of concentrated sulfuric acid were added dropwise at a temperature of 40° C. or lower over a period of 40 minutes. After 2 hours of stirring at 40° C., the system was cooled to room temperature, and 20 ml of water and 20 ml of ethyl acetate were added and stirred for 10 minutes, and the organic layer was taken. This organic layer was washed with 10 ml of water while the pH of the aqueous layer was adjusted to between 7 and 8 with 40% caustic soda, and then the organic layer was washed two more times with 10 ml of water. 10 g of propylene carbonate were added to this organic layer, and then the acetonitrile and ethyl acetate were distilled off under reduced pressure at 100° C. or lo...
example 3
[0085] 10.12 g (96% yield) of a slightly yellowish solid were obtained in the same manner as in Example 1, except that the diphenyl sulfoxide was replaced with 4.61 g (20.0 mmol) of 4,4′-dimethyldiphenyl sulfoxide.
[0086] This product was analyzed by 13C-NMR, IR, and HPLC, which revealed it to contain 99.1% (4-phenylthiophenyl)-4,4′-dimethyldiphenylsulfonium hexafluorophosphate, 0.4% thiodi-p-phenylenebis(4,4′-dimethyldiphenylsulfonium)bishexafluorophosphate, and 0.2% diphenyl sulfide and 0.3% 4,4′-dimethyldiphenyl sulfoxide as unreacted starting materials.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com