Process of preparing microspheres for sustained release having improved dispersibility and syringeability
a microsphere and dispersibility technology, applied in the direction of capsule delivery, drug composition, peptide/protein ingredients, etc., can solve the problems of reducing the stability of formulations, affecting health, and the phase separation method is problematic, and achieves the effect of high drug encapsulation efficiency, convenient mass production, and uniform size suitable for injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
Preparation of Microspheres and a Post-Process Using Various Dispersing Agents
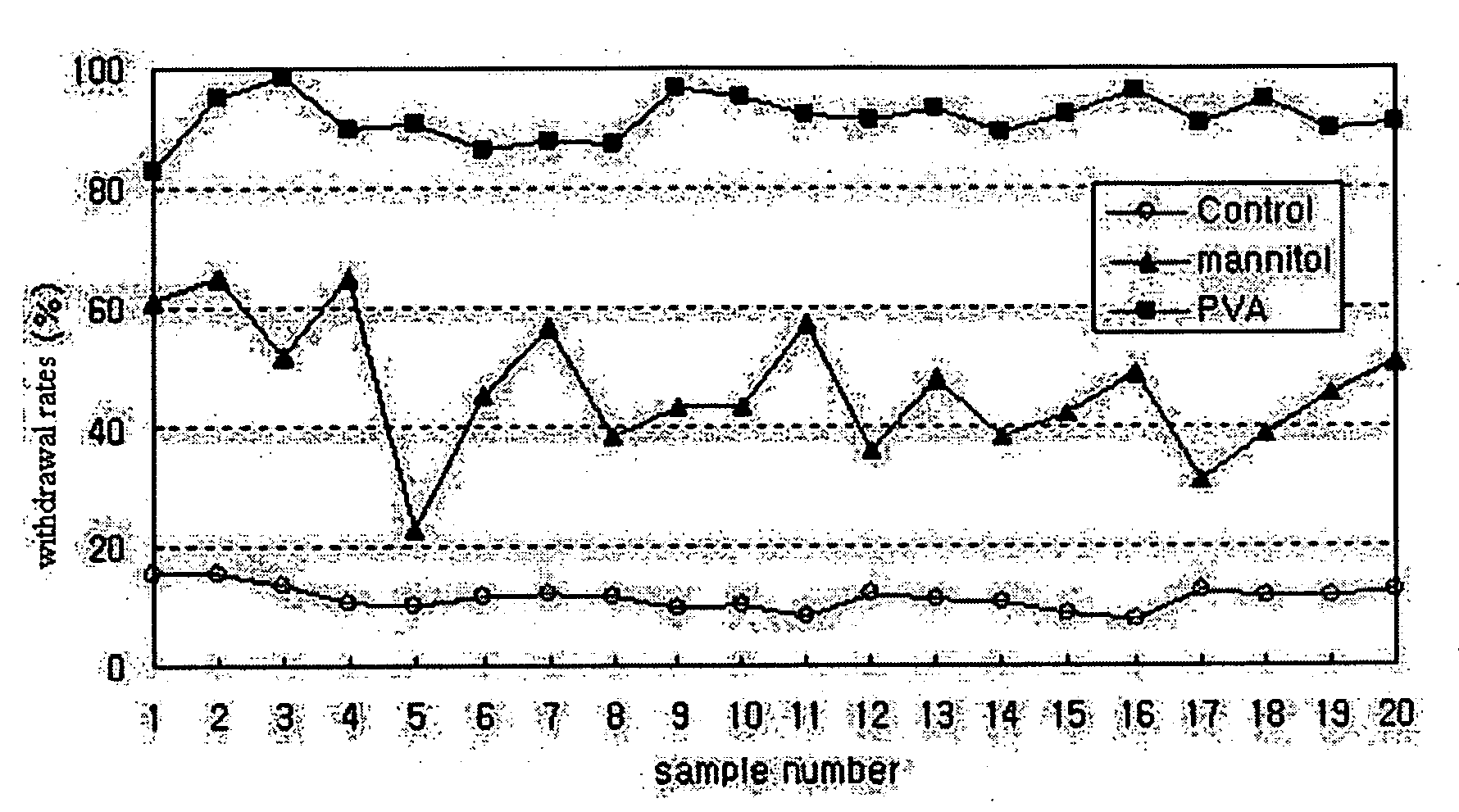
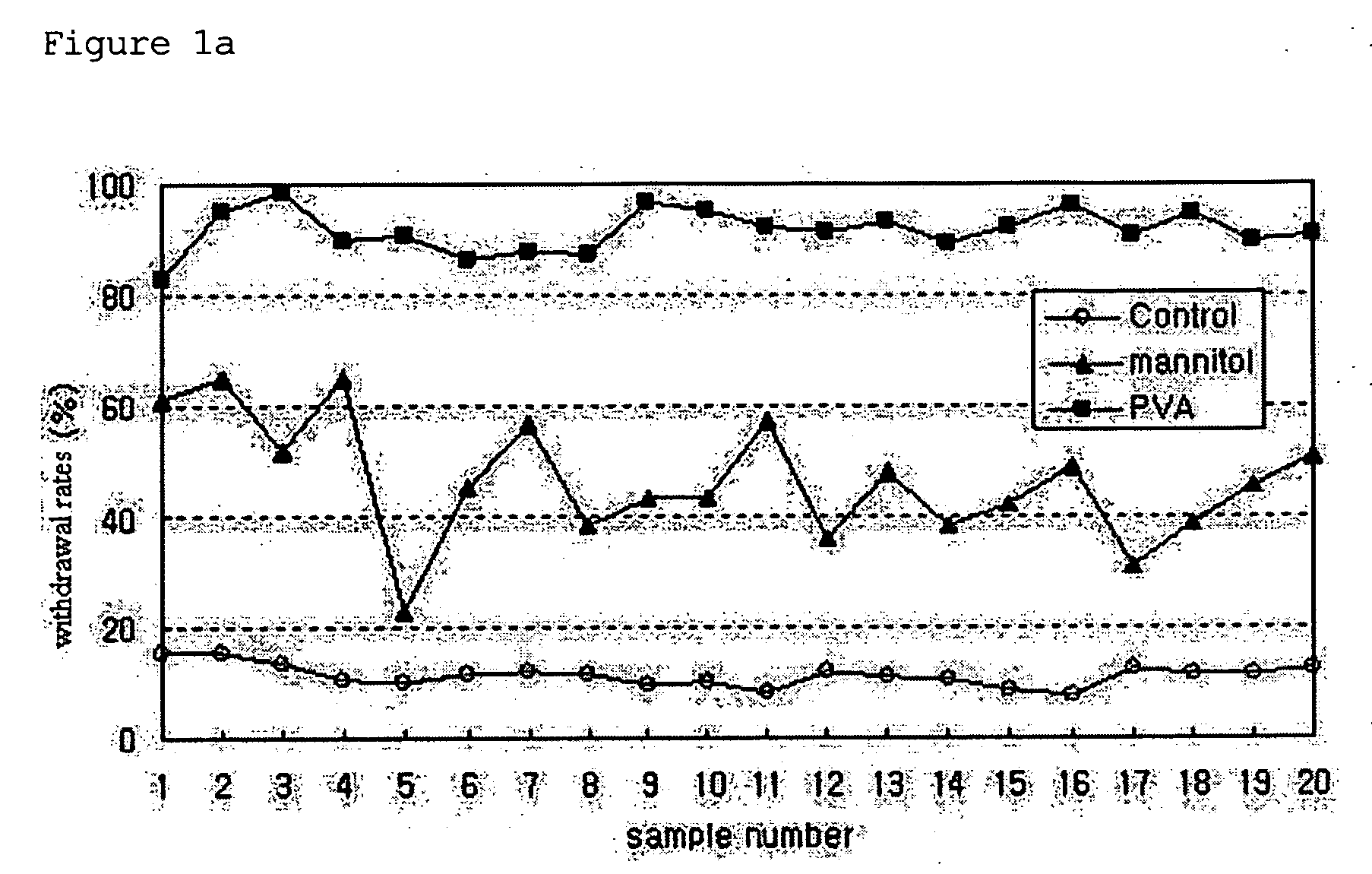
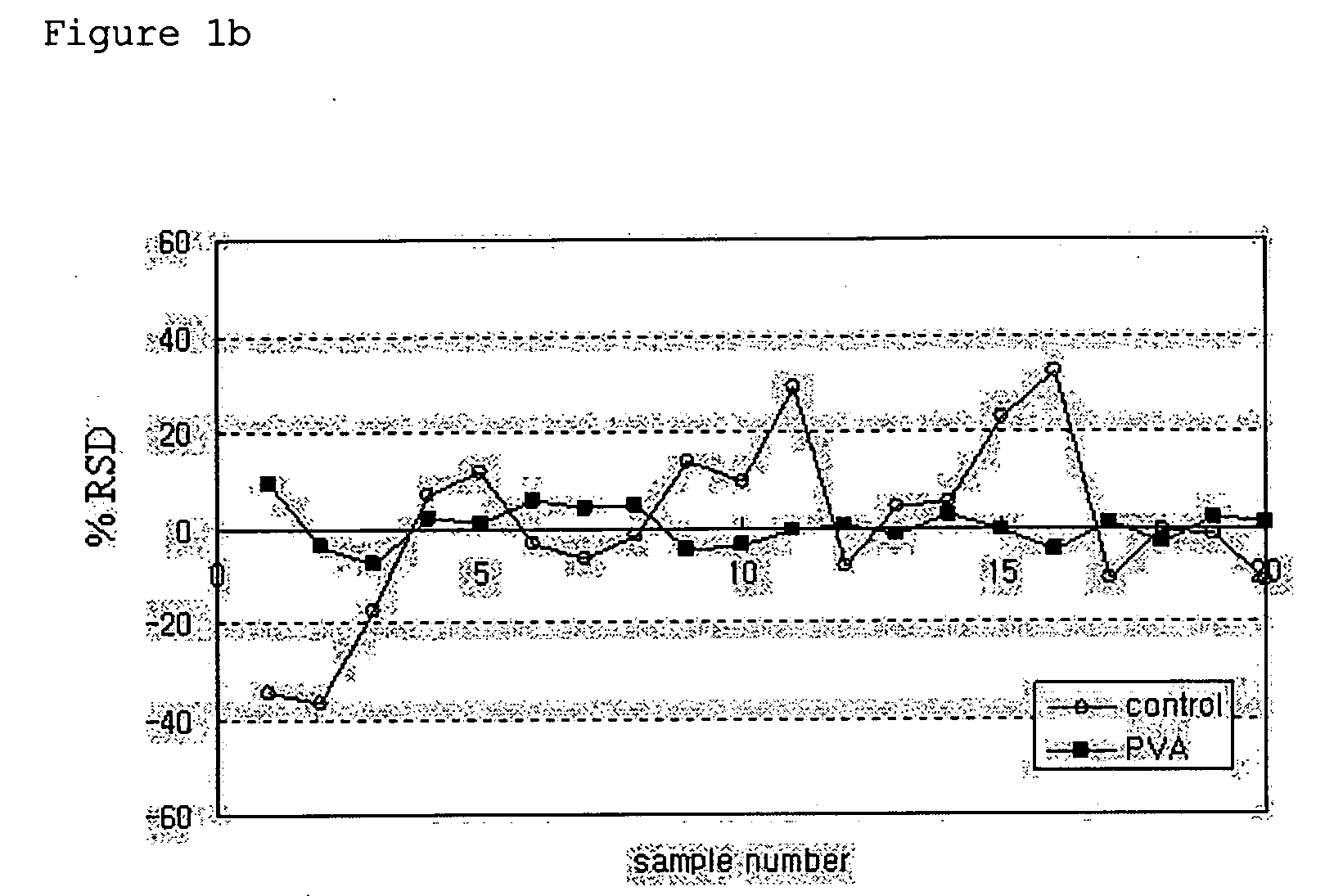
[0034]PLGA microspheres were prepared using a spray dryer (SODEVA, France) equipped with an ultrasonic nozzle (Sono-Tek, 120 kHz). A biodegradable polymer, RG503H (Boehringer-Ingelheim, Germany), and a drug, leuprolelin acetate (Polypeptide Laboratories, Denmark), were used. 50 g of RG503H and 2.5 g of leuprolelin acetate were homogeneously dissolved in 500 ml of glacial acetic acid (Yakuri Pure Chemicals, Japan). The solution was transported at a flow rate of 3 ml / min using a piston pump. The transported solution was sprayed into the spray drier through an ultrasonic nozzle installed in an upper part of a sprayer, and dried with dry air at 200° C. Thereafter, microspheres recovered from a cyclone were taken in a certain volume, weighed precisely, added at a concentration of 50 mg / ml to an aqueous solution containing distilled water and 1% (w / v) of a dispersing agent, and suspended therein for one hour at ...
preparation example 2
An Effect of Dispersal Time for Improving the Dispersibility of Microspheres
[0037]PLGA microspheres were prepared using a spray dryer (SODEVA, France) equipped with an ultrasonic nozzle (Sono-Tek, 120 kHz). A biodegradable polymer, RG503H, and a drug, leuprolelin acetate, were used. 40 g of RG503H and 4 g of leuprolelin acetate were homogeneously dissolved in 400 ml of glacial acetic acid. The solution was sprayed into a spray drier through an ultrasonic nozzle at a flow rate of 3 ml / min, and dried with dry air at 200° C. Thereafter, microspheres recovered from a cyclone were taken in a predetermined amount, added at a concentration of 50 mg / ml to an aqueous solution containing 1% (w / v) polyvinyl alcohol, and suspended therein for 1 min, 3 min, 5 min, 10 min, 1 hr, 3 hr and 6 hr at 25° C. using a magnetic agitator. The suspension was passed through a vacuum filter. Microspheres thus collected were washed with distilled water two times, and freeze-dried.
preparation example 3
Preparation of BSA-Loaded Microspheres by Spray Drying of W / O Type Emulsion Containing BSA
[0050]0.5 g of bovine serum albumin (BSA) (Sigma, A-7638) was dissolved in distilled water, and homogeneously mixed with a solution prepared by dissolving 9.5 g of RG502H in 95 ml of methylene chloride, thereby yielding a W / O type emulsion. While the emulsion was maintained in an emulsion state using an agitator, it was supplied to a spray drier (Buchi-191) at a flow rate of 3 ml / min. Compressed air was supplied to a two-fluid nozzle at a flow rate of 450 NL / h to dry sprayed atomized droplets using dry air at 80° C. The recovered microspheres were suspended for 3 hrs in an aqueous solution of 1% polyvinyl alcohol at a concentration of 50 mg / ml with agitation using a magnetic agitator, washed with distilled water, and freeze-dried. The microspheres thus prepared had a mean particle size of 5.2 μm, and the amount of polyvinyl alcohol remaining on the surface of the microspheres was 0.93% (w / w).
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersal time | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com