Novel catalyst for oxygen reduction reaction in fuel cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
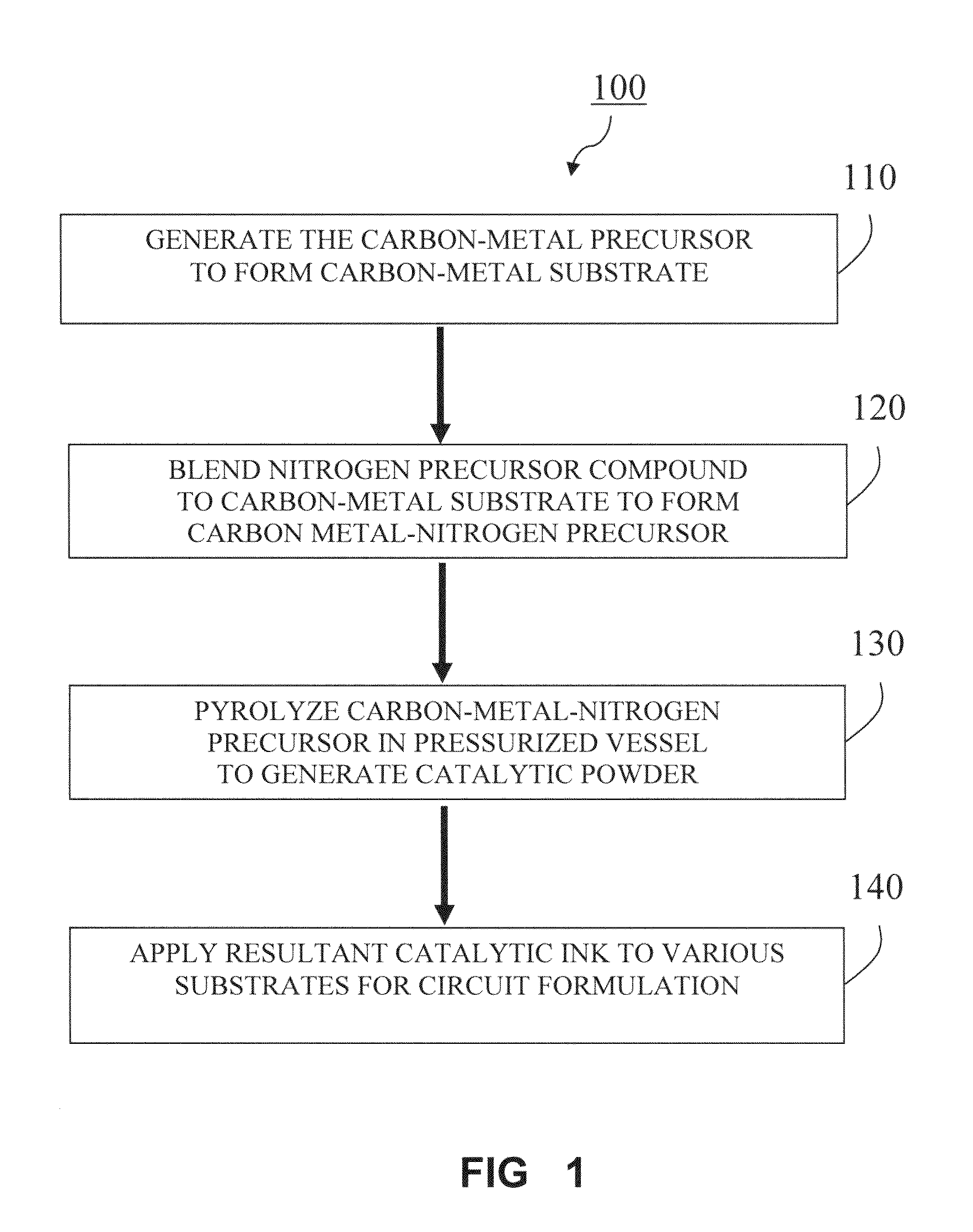
Production of Carbon-Fe-Pyridine Oxygen Reduction Cathode Catalyst
[0072]Nafion® solution (1100 EW, 5 wt. %) were purchased from Alfa Aesar, (Ward Hill, Mass., USA). A 5 mm glassy carbon rotating disk electrode (“RDE”) was purchased from Pine Instruments (Grove City, Pa., USA). Ketjenblack® 600JD (Akzo-Nobel Polymer Chemicals, Chicago, Ill., USA) (CAS No. 1333-86-4) is used as carbon support, which is dispersed in 95% ethanol. To this solution, Iron (II) acetate corresponding to 1 wt. % of iron on carbon is added and the slurry is kept stirring for about 6 hr. After the solvent is evaporated and a dry composite powder is obtained, 55 mg of the composite material thus obtained is ground with varying amounts of 2,2′ bipyridine ranging from 35 to 85 mg and the powder is subsequently charged into a stainless steel bomb that has a volume of 1.7 ml. The pyrolysis vessel (bomb) can be a closed vessel made from steel, ceramics or quartz. The material was charged into the bomb in an inert atm...
example 2
Electrochemical Evaluation of the Cathode Catalyst Performance
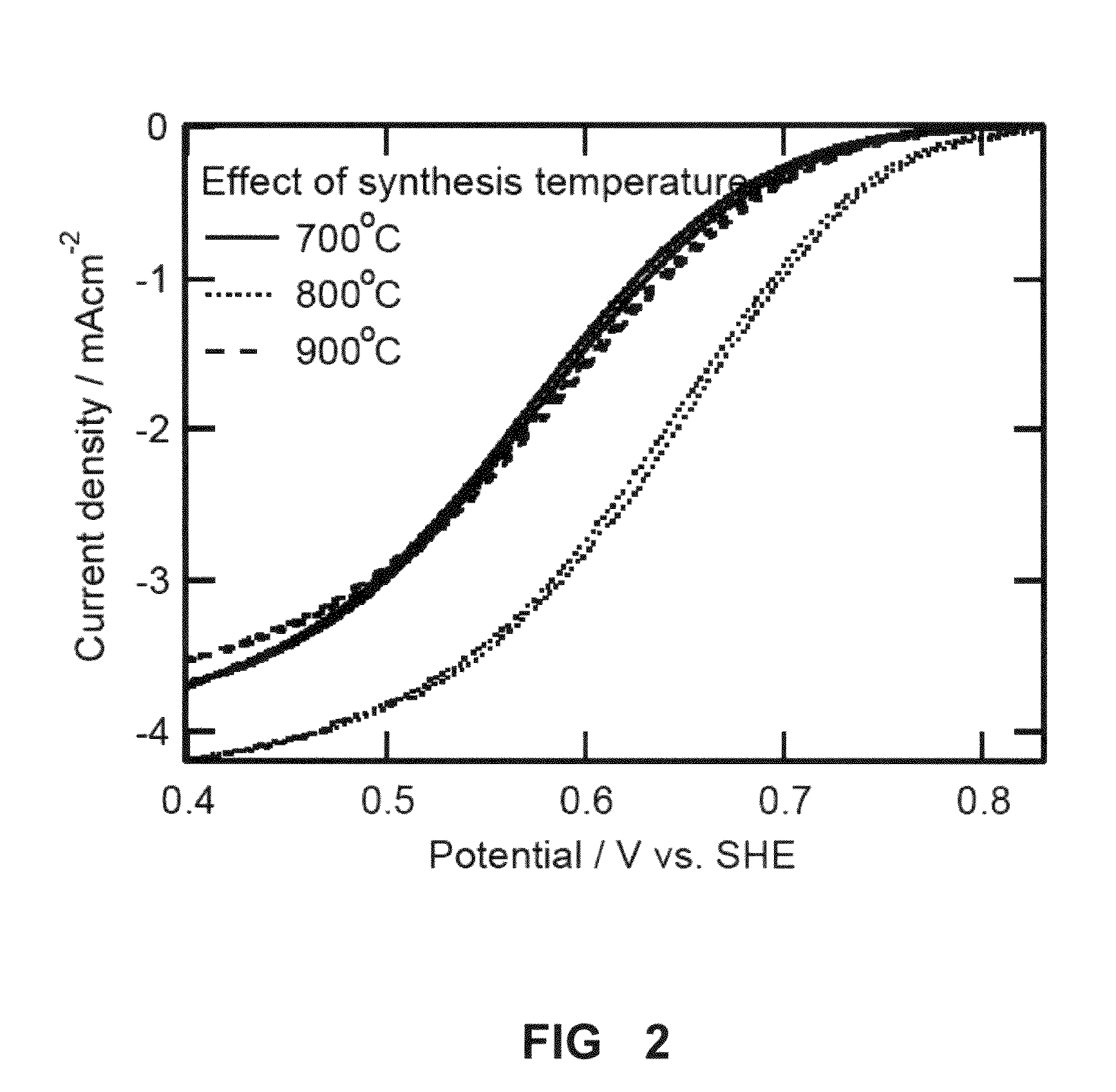
[0073]The catalysts thus obtained were tested in a rotating disc electrode set up, using 1N H2SO4 as the electrolyte at 40° C. As will be appreciated by those of ordinary skill in the art, the rotating disk electrode (RDE) consists of a disk on the end of an insulated shaft that is rotated at a controlled angular velocity. Providing the flow is laminar over the entire disk, the mathematical description of the flow is surprisingly simple, with the solution velocity towards the disk being a function of the distance from the surface, but independent of the radial position. The rotating disk electrode is used for studying electrochemical kinetics under conditions, such as those of testing the present technology, when the electrochemical electron transfer process is a limiting step rather than the diffusion process. Hg / Hg2SO4 was used as the reference electrode for all the studies and a platinum wire serves as the counter elec...
example 3
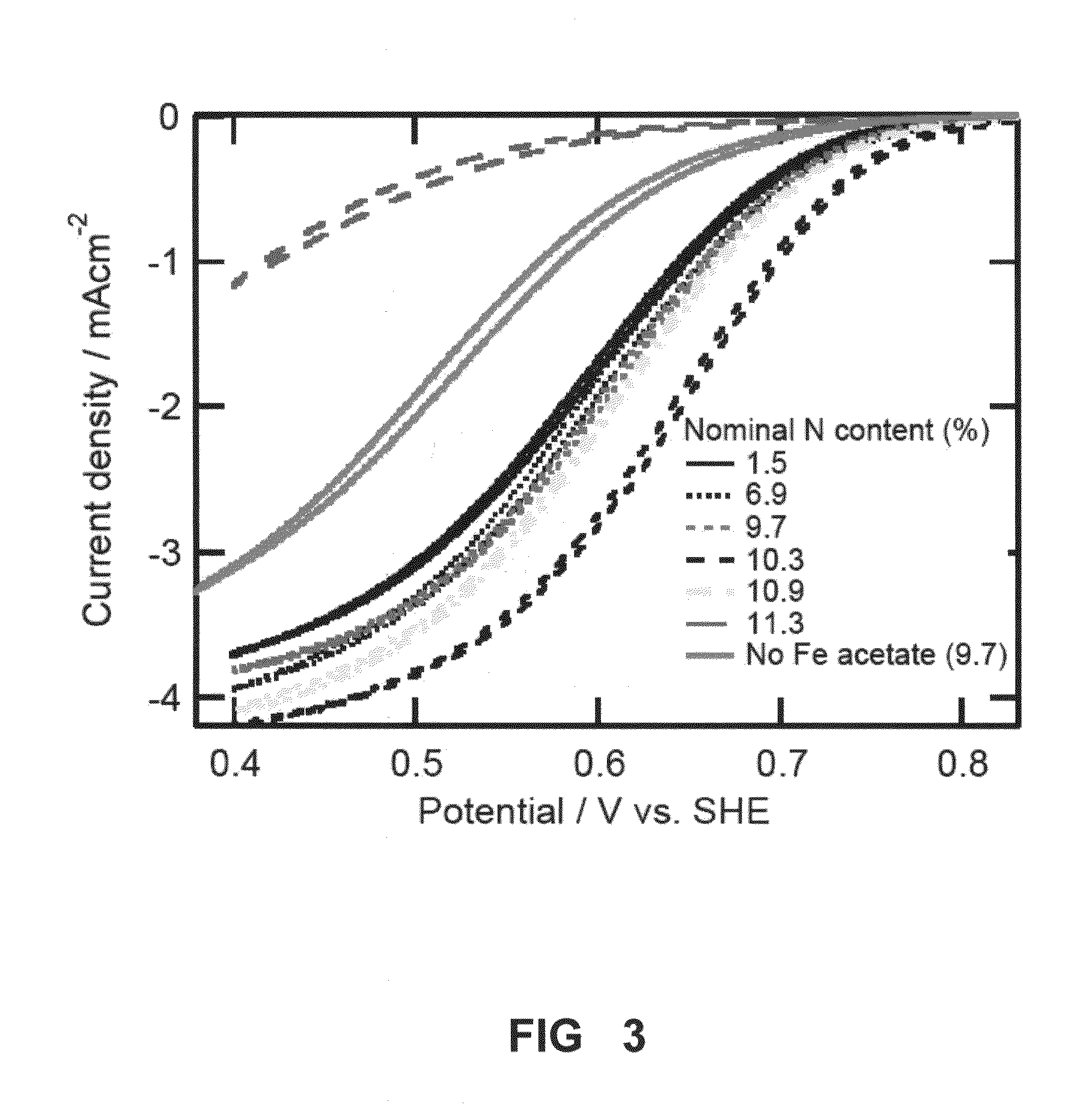
The Nitrogen / Carbon (N:C) Ratio Affect on Cathode Catalyst Performance
[0082]As mentioned above, the Nitrogen / Carbon (N:C) ratio demonstrates an important property of nitrogen precursors for metal-nitrogen-carbon (MNC) catalysts. Increasing the N:C ratio of the nitrogen precursor increases the accessible active site density by reducing carbon deposition in the pores of the carbon support during pyrolysis.
[0083]In one example, Ketjenblack® 600JD is dispersed in a 95% ethanol solution, to which iron (II) acetate corresponding to 0.75 wt % Fe is added. This slurry is stirred for 6 hr followed by solvent evaporation to yield a dry powder. Powder samples of 55 mg are ground with varying amounts of pyridinic nitrogen rich precursors, such as bipyridine (having a N:C ratio of 0.2), pyrazine (N:C=0.5), purine (N:C=0.8), and melamine (N:C=2.0) to achieve nominal 6.3 wt % nitrogen loading. The powder is subsequently charged into a 1.7 mL quartz ampule. The ampule is flame-sealed under vacuum a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com