Resist underlayer film forming composition for lithography containing polyether structure-containing resin
a technology of polyether structure and composition, applied in the direction of plastic/resin/waxes insulators, instruments, photomechanical equipment, etc., can solve the problem of large influence of active ray diffused reflection on the substrate or standing wave, difficult to secure a resist pattern film thickness sufficient for processing the substrate, and reduce the effect of etching resistan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Synthesis Example 1
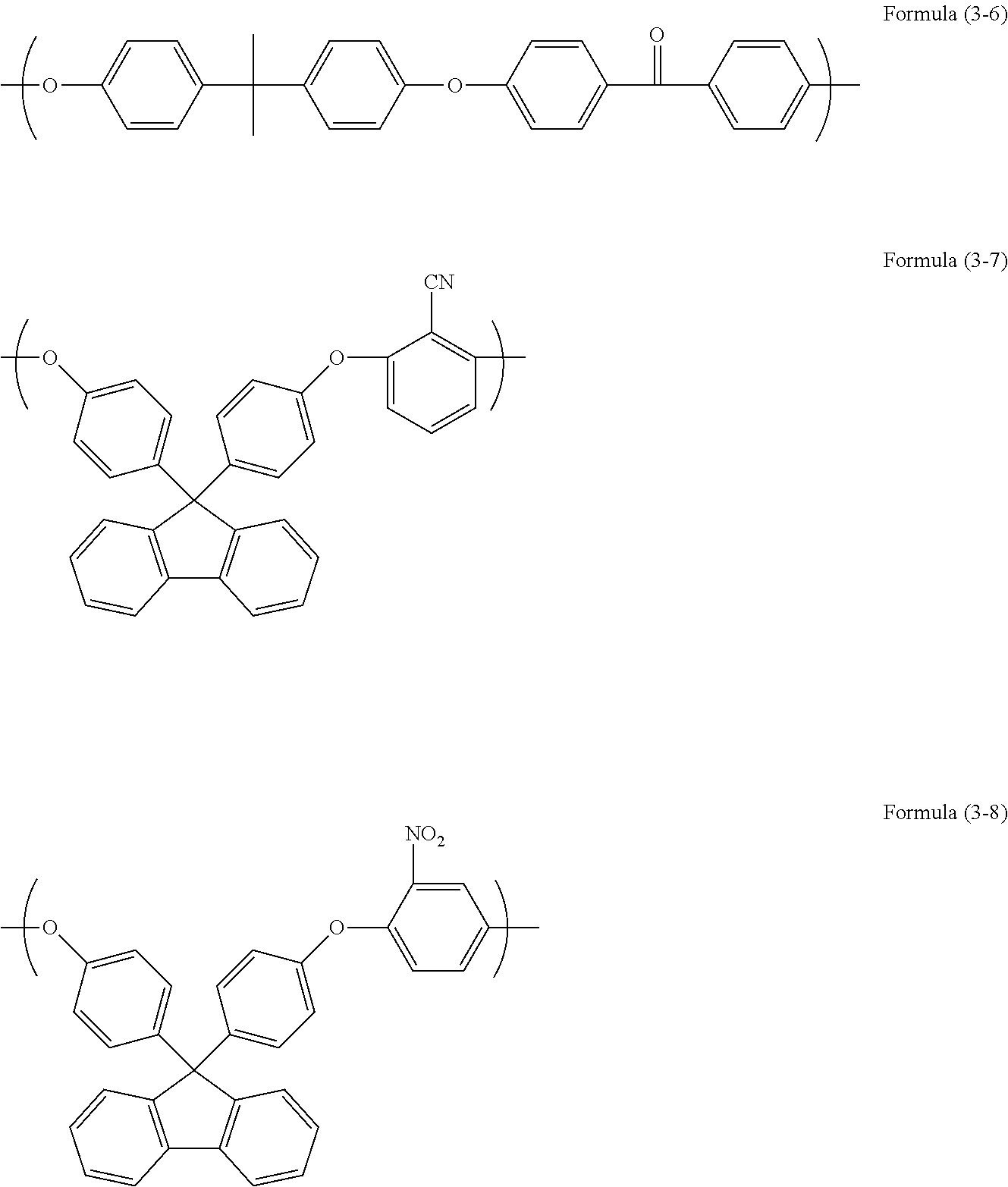
[0071]Into a flask equipped with a stirrer, a reflux apparatus, and a thermometer, 28.04 g of 9,9-bis(4-hydroxyphenyl)fluorene, 13.97 g of 4,4′-difluorobenzophenone, 12.32 g of potassium carbonate, and 162.56 g of N-methyl-2-pyrrolidinone were charged. Then, the inside of the flask was purged with nitrogen and the flask was heated until the inner temperature thereof became 140° C., followed by effecting the reaction for about 24 hours. The synthesized polymer was cooled down to room temperature and the reaction mixture was filtered for removing a precipitate to recover the resultant reaction filtrate. The reaction filtrate was mixed with about 10 mL of a mixture of N-methyl-2-pyrrolidinone and 2 mol / L hydrochloric acid in a volume ratio of 90:10. Then, the resultant reaction filtrate was charged into methanol to perform reprecipitation purification of the reaction filtrate.
[0072]Furthermore, the resultant precipitate was washed with water and methanol and was vacu...
synthesis example 2
[0073]Into a 100 mL three-neck flask, 6.76 g of 6,6′-(9H-fluorene-9,9-diyl)dinaphthalene-2-ole, 3.27 g of 4,4′-difluorobenzophenone, 42.72 g of N-methyl-2-pyrrolidinone, and 2.49 g of potassium carbonate were charged. Then, the inside of the flask was purged with nitrogen and the flask was heated to 170° C., followed by effecting the reaction for about 24 hours. Then, to the resultant reaction mixture, 0.65 g of 1-naphthol dissolved in 5.84 g of N-methyl-2-pyrrolidinone was added and the resultant reaction mixture was stirred further for 2 hours. After the completion of the reaction, the reaction mixture was diluted with 20 g of N-methyl-2-pyrrolidinone and a precipitate was removed by filtration. The recovered filtrate was dropped into a mixed solution of methanol / water / toluene (350 g / 50 g / 30 g) to perform reprecipitation. The resultant precipitate was filtered under reduced pressure and the filtered substance was dried under reduced pressure at 85° C. over one night. Then, 7.92 g ...
synthesis example 3
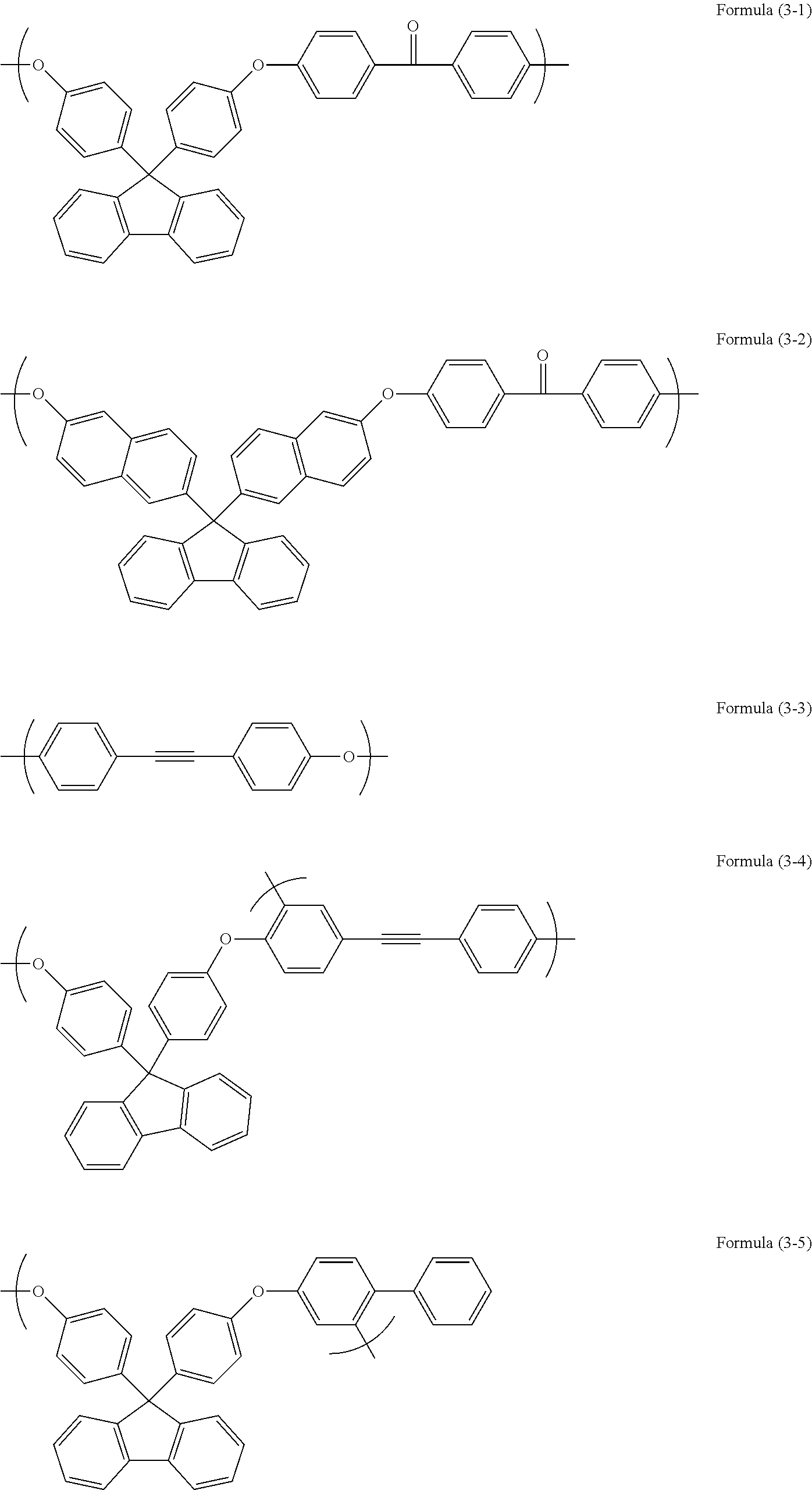
[0074]Into a 100 mL three-neck flask, 5.09 g of 4-(4-fluorophenylethynyl)phenol, 45.84 g of N-methyl-2-pyrrolidinone, and 3.65 g of potassium carbonate were charged. Then, the inside of the flask was purged with nitrogen and the flask was heated to 170° C., followed by effecting the reaction for about 24 hours. After the completion of the reaction, a precipitate was removed by filtration. The recovered filtrate was dropped into 400 g of methanol to perform reprecipitation. The resultant precipitate was filtered under reduced pressure and the filtered substance was dried under reduced pressure at 85° C. over one night. Then, 5.12 g of a polyether was obtained as a green color powder. The obtained polymer corresponded to Formula (3-3). By GPC, the obtained polymer had a weight average molecular weight Mw of 51,000 and a polydispersity Mw / Mn of 5.47 that were measured in terms of polystyrene.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com