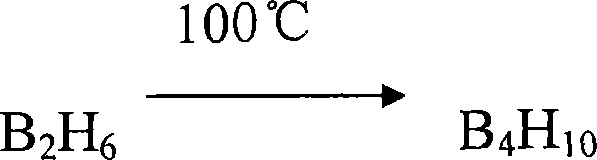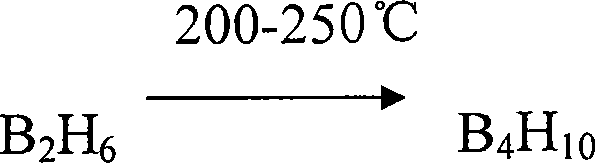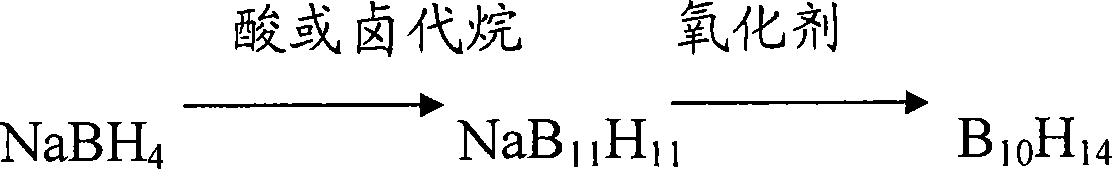Compounds used for element forming volatile matter
A technology for compounds and volatiles, applied in the field of instrumental analysis, can solve the problems of insufficient theoretical expression, failure to specify the molecular formula and preparation method of reagents, obstacles, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] According to the usual synthesis method, after the dodecaborane disodium salt is synthesized by sodium borohydride, the product dodecaborane disodium salt is formulated into a 1.2% aqueous solution, and then the solution is continuously mixed online with a peristaltic pump and elements containing gold, Silver, copper, platinum, ruthenium, palladium, iridium sample solution (element content is 100ppb, nitric acid acidity 0.6M) the mixed solution is separated in a gas-liquid separator, and the volatile gas hydride generated is sent to When detected by inductively coupled plasma emission spectroscopy, the sensitivity of the measured elements is 80-90 times that of dodecaborane disodium salt in solution under the same conditions.
Embodiment 2
[0056] According to the usual synthesis method, after synthesizing undecaborane monosodium salt by sodium borohydride, the product is formulated into a 1.2% aqueous solution, and then the solution is continuously mixed online with a peristaltic pump to contain elements cobalt, nickel, manganese, vanadium, The sample solution of yttrium, zirconium, and scandium (the element content is 100ppb, and the acidity of nitric acid is 0.2M) separates the mixed solution in a gas-liquid separator, and the generated volatile gas hydride is sent to the inductively coupled plasma emission by the carrier gas Detection in the spectrum, the sensitivity of the measured elements is 100-150 times that under the same conditions when the solution does not contain undecaborane monosodium salt.
Embodiment 3
[0058] Synthesize KB according to the usual synthetic method 3 h 8 (potassium triboron octahydrogenate), this product is formulated into 1.2% aqueous solution, then continuously mixes this solution and the sample solution containing element cobalt, nickel, manganese, vanadium, yttrium, zirconium, scandium with peristaltic pump online (element content Both are 100ppb, and the acidity of nitric acid is 0.2M) the mixed solution is separated in a gas-liquid separator, and the volatile gas hydride generated is sent to the inductively coupled plasma emission spectrum by the carrier gas for detection, and the measured element sensitivity is Under the same conditions, the solution does not contain KB 3 h 8 (Potassium triboroctahydrogenate) 100-120 times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com