Method for synthesizing formic ester and specific catalyzer thereof
A technology of catalyst and formate, which is applied in the direction of physical/chemical process catalyst, carbon monoxide or formate reaction preparation, metal/metal oxide/metal hydroxide catalyst, etc., can solve the problem of improving process cost, space-time yield and Low selectivity, high synthesis cost of esterification method, etc., to achieve huge economic and social benefits, high conversion efficiency and selectivity, and wide industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1, synthetic methyl formate
[0019] Place 0.5mmol of copper nitrate and 50.0mmol of polyvinylpyrrolidone (PVP) in the reaction flask successively, add 20.0mL of methanol, stir, and dropwise add 10.0mL of 0.5mol / L NaBH 4 Methanol solution was reacted at room temperature for 30 minutes to obtain a copper nanoparticle catalyst.
[0020] The catalyst was placed in a 100mL reactor, filled with 3.0MPaCO, and reacted at 150°C for 12 hours.
[0021] The conversion rate of CO was 45%, and the selectivity of the target product methyl formate detected by GC / MS was 100%.
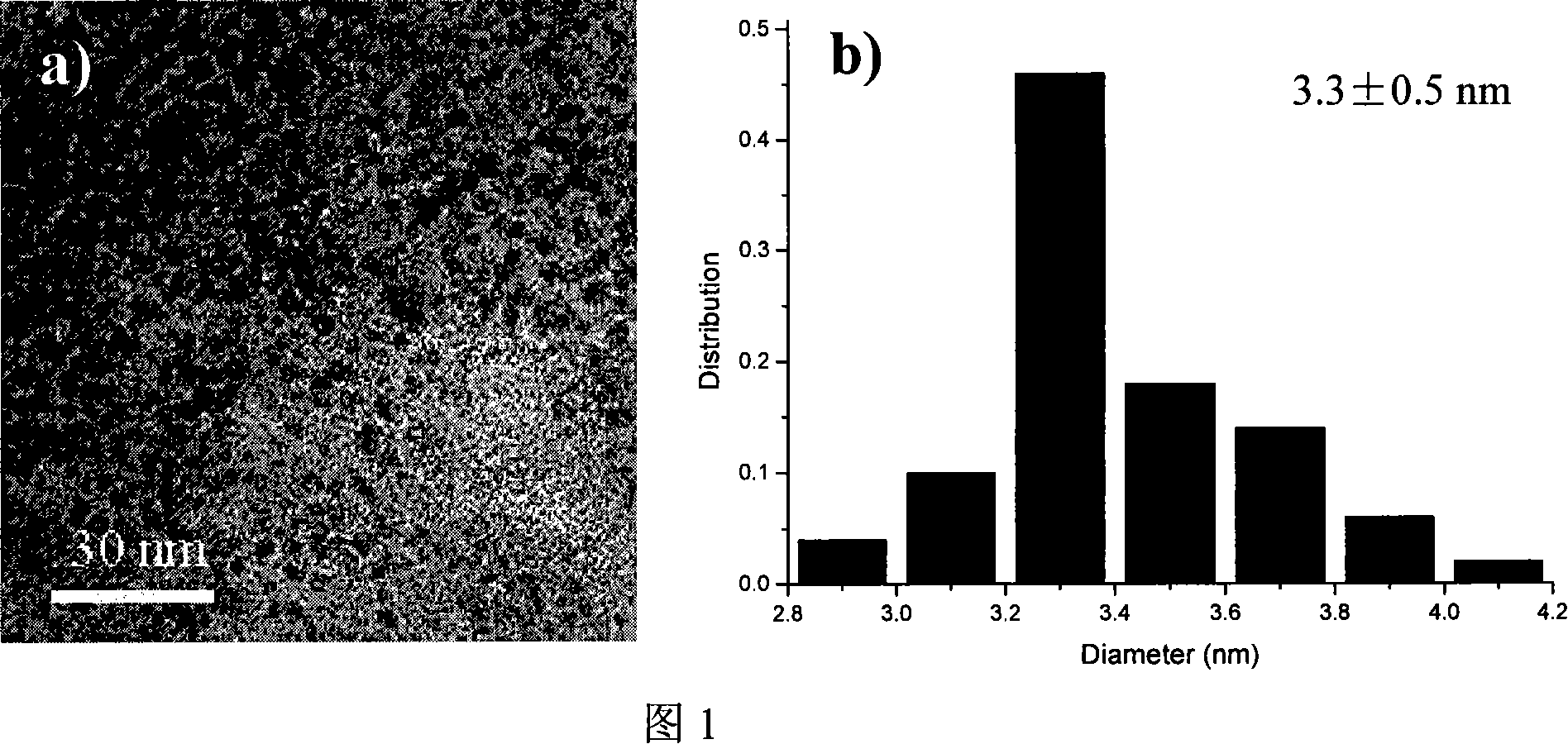
[0022] The photo of the copper nanoparticles observed by the electron microscope is shown in Figure 1a, and the particle size distribution is shown in Figure 1b. It can be seen from the figure that the particle size distribution of the nanoparticles is relatively narrow, about 3.3±0.5nm. Utilizing the catalyst to synthesize methyl formate can obtain a high CO conversion rate of 45%, and the selecti...
Embodiment 2
[0023] Embodiment 2, synthetic methyl formate
[0024] Put the metal salt copper chloride and PVP in the reaction flask at a molar ratio of 1:50, add 30mL of methanol, stir, and dropwise add 10mL of 0.1mol / L NaBH 4 Methanol solution, react at room temperature for 0.5 hour to obtain nanometer particle catalyst, the particle diameter of which is 2-4nm.
[0025] The catalyst was placed in a 100mL reactor, filled with 0.3MPaCO, and reacted at 170°C for 16 hours.
[0026] The conversion rate of CO was 25%, and the selectivity of the target product methyl formate detected by GC / MS was 100%.
Embodiment 3
[0027] Embodiment 3, synthetic methyl formate
[0028] Put the metal salt copper acetylacetonate and PVP in a molar ratio of 1:100 successively in the reaction flask, add 30mL of methanol, stir, add dropwise 10mL of 0.1mol / L NaBH 4 The methanol solution is reacted at room temperature for 1 hour to obtain a nanoparticle catalyst with a particle size of 2-4nm.
[0029] The catalyst was placed in a 100mL reactor, filled with 2.0MPaCO, and reacted at 130°C for 8 hours.
[0030] The conversion rate of CO was 38%, and the selectivity of the target product methyl formate detected by GC / MS was 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com