Organic solvent resisting basified protease producing strain, gene and application thereof
A technology resistant to organic solvents and proteases, applied in applications, genetic engineering, plant genetic improvement, etc., can solve the problems of protease loss of activity and low stability, and achieve strong solvent tolerance, high specific activity, and a wide range of pH Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This experiment illustrates the screening procedure for natural strains producing organosolv-resistant proteases.
[0034] Organic solvent-resistant extremophiles were screened from oily soil samples and other samples using different concentrations of cyclohexane, toluene, acetone and other organic solvents as the selection pressure. Using SMA medium, the specific formula is: skimmed milk powder 12g / L, nutrient agar 13.8g / L, inoculate organic solvent-resistant extremophiles on SMA plates, and obtain strains that can produce high-activity protease according to the ratio of colony to transparent circle size. Eight strains of organic solvent-resistant extremophiles with higher protease activity were screened by this method.
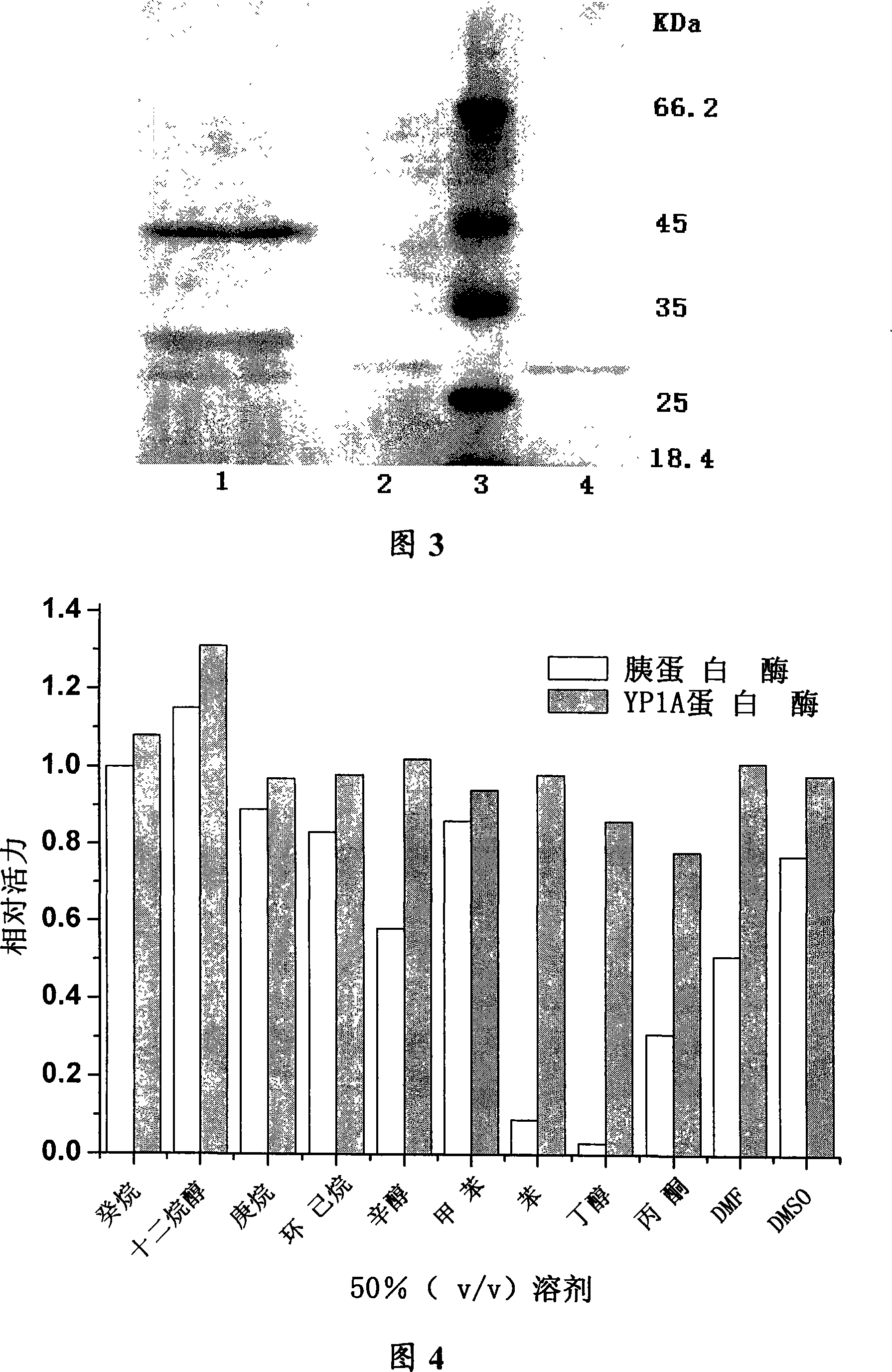
[0035] In order to further test the solvent tolerance of the secreted proteases, the protease activity of the eight strains and the organic solvent resistance of the proteases produced were comprehensively tested. Inoculate the producing bacteria wit...
Embodiment 2
[0038] This experiment illustrates the biological properties of organic solvent-resistant extremophile Bacillus licheniformis YP1A.
[0039] Physiological and biochemical properties
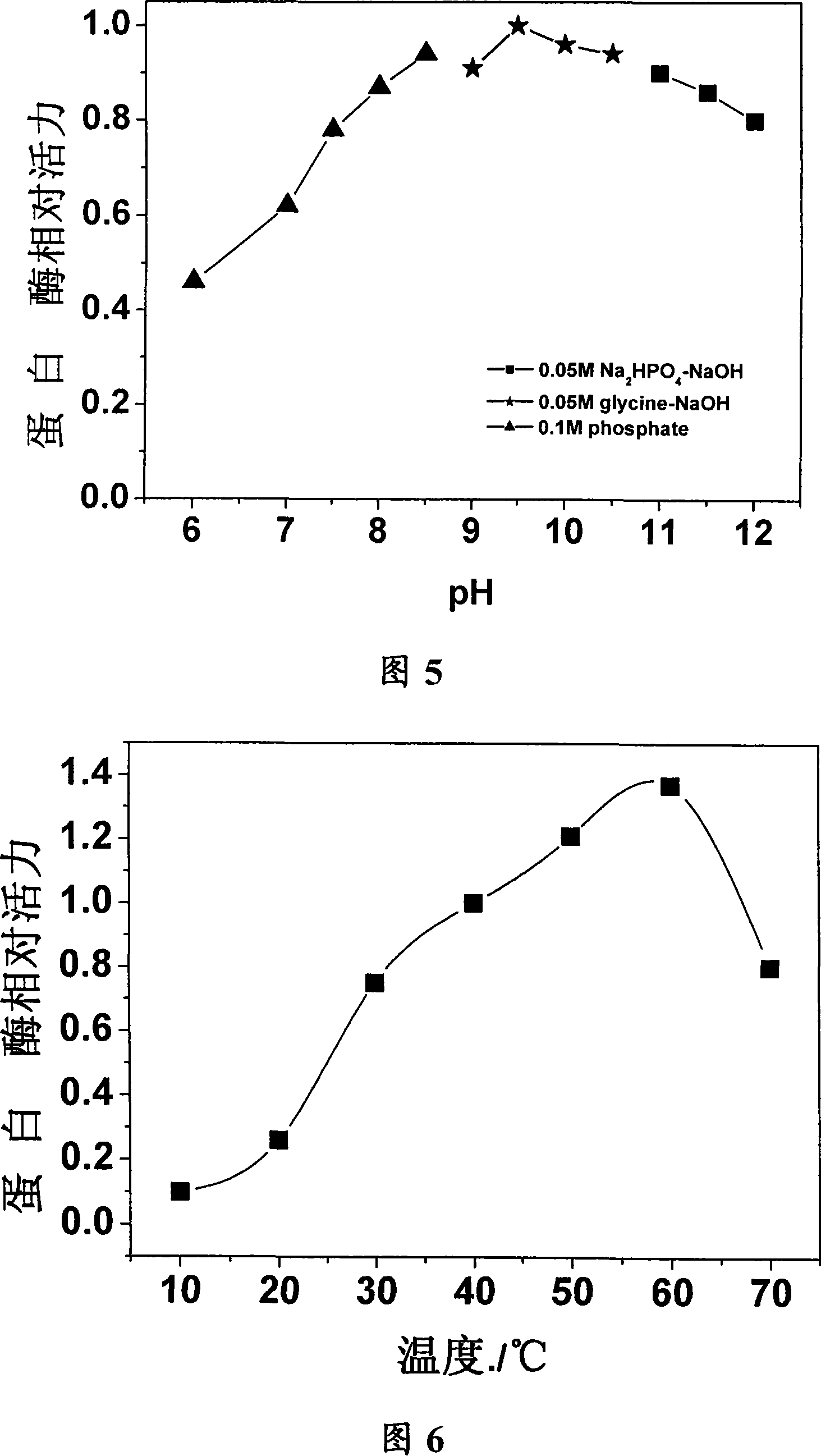
[0040] Gram stain observation of the bacteria indicated that the strain was G + Bacillus, with spores, observed by transmission electron microscope showed that the strain had pericytic flagella, the size of which was 0.5μm×2~3μm. After growing in broth medium for 14 hours, the colony size is 1.5-2 mm, the growth temperature range is 25-42 °C, the optimum temperature is 35 °C, the growth pH is 7-12, and the optimum pH is 8.5. Its physiological and biochemical characteristics It is shown that the utilization of citric acid, D-xylose and mannitol is positive for the contact enzyme reaction, and the utilization of D-arabinose, α-D-lactose and D-galactose is negative, and it grows under aerobic conditions. Part of the physiological and biochemical identification experimental characteristics of the b...
Embodiment 3
[0046] This experiment illustrates the purification procedure for organosolv-resistant proteases.
[0047] First, precipitation of ammonium sulfate resistant to organic solvent protease was carried out. After the strain was cultured in the enzyme production medium for 48 hours, the supernatant enzyme solution was taken by centrifugation. The crude enzyme solution was placed in an ice bath, and ammonium sulfate was slowly added to 80 ° % Saturation, let stand overnight at 4°C. Then centrifuge at 13,000 rpm for 30 min, and add the enzyme solution obtained by 80% ammonium sulfate precipitation to a solution containing 1 mol (NH 4 ) 2 SO 4 , 50mmol / L Glycine-NaOH buffer A (pH9.0) equilibrated Phenyl sepharose CL-4B chromatography column, using A, B buffer mixture for stage elution (buffer B is 50mmol / L Glycine-NaOH, pH9.0). Protease activity was present in fractions collected at 50% B. with SP Sepharose Tm For FF strong cation exchange chromatography, the pH of the upper co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com