Method for catalytically synthesizing adamantane
A technology of adamantane and main catalyst, applied in the direction of organic chemistry, isomerization hydrocarbon production, etc., can solve the problems of low yield and increased cost, and achieve the effect of cheap and easy-to-obtain raw materials, easy control, and simple and easy experimental methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
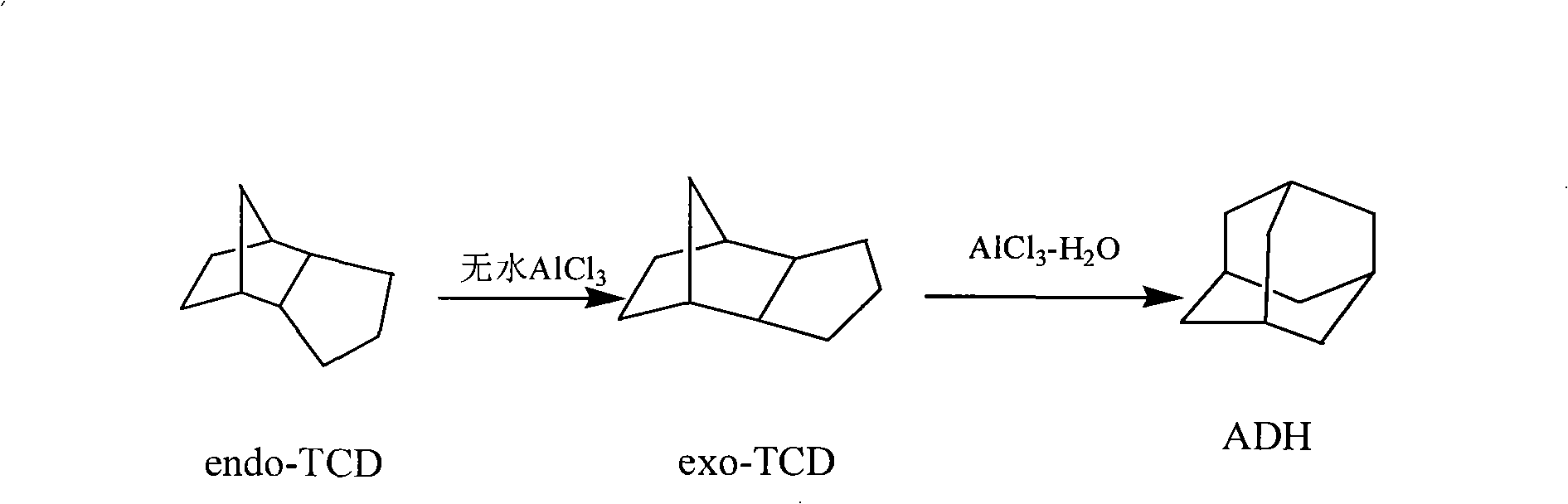
[0019] A method for catalytically synthesizing adamantane, the steps are:
[0020] A. Isomerization into pendant tetrahydrodicyclopentadiene (exo-TCD): add bridged tetrahydrodicyclopentadiene 13.6g, anhydrous aluminum trichloride (main catalyst) 2.0g in a 250ml three-necked flask, dry (Anhydrous CaCl 2 ) over 2.4ml of 1,2-dichloroethane, start stirring to dissolve bridged tetrahydrodicyclopentadiene slowly, the reaction temperature is 20°C or 25°C, the reaction time is 30min, and the reaction product is directly used in the next step reaction;
[0021] B, the synthesis of adamantane (ADH): after the above reaction is completed, add 3.0g anhydrous aluminum trichloride and 0.20g water, the reaction temperature is 60°C or 70°C, and the reaction time is 3 hours. Add 30ml petroleum ether into the flask, stir, and after the adamantane is completely dissolved, let stand for 10min. Pour the supernatant into a 100ml beaker, place it in an ice-water bath to cool, and keep the tempera...
Embodiment 2
[0023] In a 250ml three-necked flask, add bridged tetrahydrodicyclopentadiene 16g, anhydrous aluminum trichloride 2.5g, dry (anhydrous CaCl 2 ) over 3.6ml of 1,2-dichloroethane, start stirring to slowly dissolve the bridged tetrahydrodicyclopentadiene, the reaction temperature is 30°C or 35°C, the reaction time is 40min, then add 3.0g of anhydrous trichloro Aluminum and 0.30g sodium bicarbonate, the reaction temperature is 60°C or 65°C, and the reaction time is 3.5 hours. After the reaction, add 40ml of n-hexane into the three-necked flask, stir, and let the adamantane dissolve completely for 10 minutes. Pour the supernatant into a 100ml flask, and evaporate until the adamantane is completely crystallized. After the adamantane is air-dried at room temperature for 4 hours, a white crystalline adamantane with a purity of 99% and a melting point of 269°C (sealed tube) can be obtained. 10g, the yield of adamantane is 62.5%.
Embodiment 3
[0025] In a 250ml three-necked flask, add 20g of bridged tetrahydrodicyclopentadiene, 4.0g of anhydrous aluminum trichloride, and 4.0ml of dried 1,2-dichloroethane, and start stirring to make bridged tetrahydrodicyclopentadiene The diene dissolves slowly, the reaction temperature is 40°C or 50°C, the reaction time is 1 hour, then add 4.0g of anhydrous aluminum trichloride and 0.40g of sodium carbonate, the reaction temperature is 80°C or 90°C, the reaction time is 4 hours, and the reaction ends After that, the temperature was lowered to 40°C, 10ml of n-hexane was added into the three-necked flask, stirred, and after the adamantane was completely dissolved, it was left to stand for 10 minutes, and the extraction was repeated 3 times. The supernatant was poured into a 100ml flask, and evaporated until the adamantane was completely crystallized. After the adamantane was air-dried at room temperature for 6 hours, 13.5 g of white crystal adamantane with a purity of 99.5% was obtaine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com