A kind of fibrinolytic active protein from scorpion tail and its preparation method and application
An active protein and fibrinolytic technology, applied in the preparation of anti-tumor drugs, in the field of extraction of fibrinolytic active protein from scorpion tail, can solve the problems that there are no related reports on scorpion venom fibrinolytic active protein, and achieve easy purification and batch preparation, high specific activity, and strong fibrinolytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
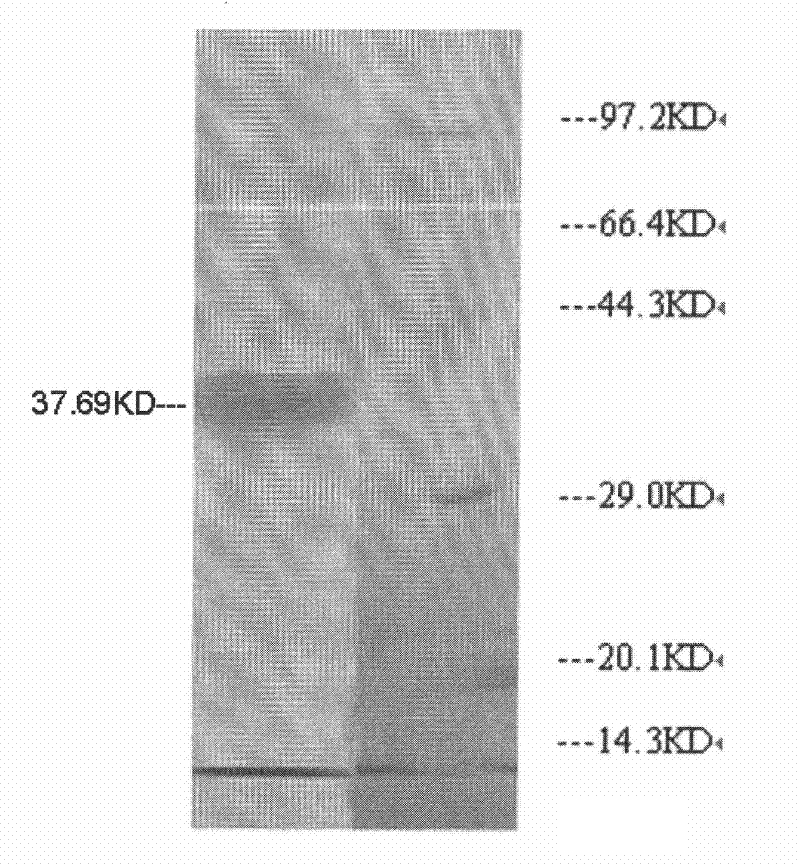
[0030] Preparation of fibrinolytic active protein from scorpion venom.
[0031] Material: East Asian scorpion (origin: China)
[0032] Preparation: 1. Preparation of crude extract
[0033] Take the scorpion, cut the scorpion tail, weigh it, cut it into pieces, add pre-cooled 0.02mol / L sodium phosphate buffer pH7.2 at a ratio of 1:5 (weight: volume), grind until it is minced, and extract overnight at 4°C . Centrifuge the extract at 8500 rpm, 4°C for 15 minutes, collect the supernatant, add solid ammonium sulfate to the supernatant to reach 40% saturation, and centrifuge at 8500 rpm, 4°C , collect the supernatant; then add solid ammonium sulfate to reach 70% saturation, 8500 rpm, centrifuge in a centrifugal pump at 4°C, collect the precipitate and carry out dialysis concentration to obtain a crude extract.
[0034] 2. Ion exchange chromatography
[0035] Pre-equilibrate the DEAE-32 cellulose column with 0.02mol / L Tris-HCl buffer solution pH8.0, gradient elution with 0.02mol / ...
Embodiment 2
[0039] Determination of biological activity of plasmin in the present invention.
[0040] Reagent:
[0041] Bovine fibrinogen solution: concentration 10mg / mL. Bovine fibrinogen is a product of Sigma.
[0042] Bovine thrombin: concentration 100U / mL. Bovine thrombin is a product of Sigma.
[0043] operate:
[0044] Prepare 0.8% agarose, boil and cool to 45-55°C, add 0.8ml of 7mg / ml bovine fibrinogen, 0.5ml of 6U / ml thrombin, shake quickly and pour into a 9cm diameter petri dish, cover Put on the glass cover, cool and solidify, move to 4°C refrigerator for half an hour, use a 0.2ml pipette tip to punch holes on the prepared fibrin gel plate, add 20 μL of sample to each hole, and incubate in a 37°C incubator for 24 hours , stained with Coomassie Brilliant Blue R-250 for 30 minutes, decolorized for 2 hours, measured the size of the two vertical diameters of the dissolution circle to calculate the area of the dissolution circle, and compared with the standard curve of urokina...
Embodiment 3
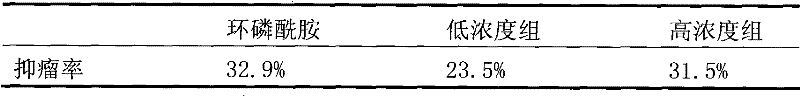
[0046] The application of the protein containing the fibrinolytic activity of the present invention in the preparation of antitumor drugs.
[0047] The inhibitory effect of this fibrinolytic active protein on mouse ascites tumor cell S180 was determined respectively, experiments related to intraperitoneal injection and axillary injection of solid tumor were designed, and the direct effect of this fibrinolytic active protein on S180 cells was detected by MTT assay.
[0048] 1) Establishment and results of the experimental model of intraperitoneal injection of S180 in mice
[0049] Inoculate mice (20±2g) with a cell density of 8×10 7 , intraperitoneal injection, each set 8 repeated groups, the positive control cyclophosphamide group concentration is 20mg / ml, the negative control group is equal volume of normal saline, and the drug concentration group is the above-mentioned scorpion tail fibrinolytic activity protein concentration group 25mg / ml , 100mg / ml, intraperitoneal injectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com