Preparation method of Agomelatine
A compound, sodium borohydride technology, applied in the preparation field of agomelatine, can solve the problems of high equipment requirements, low yield, easy spontaneous combustion, etc., and achieve the effects of simple operation, stable process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
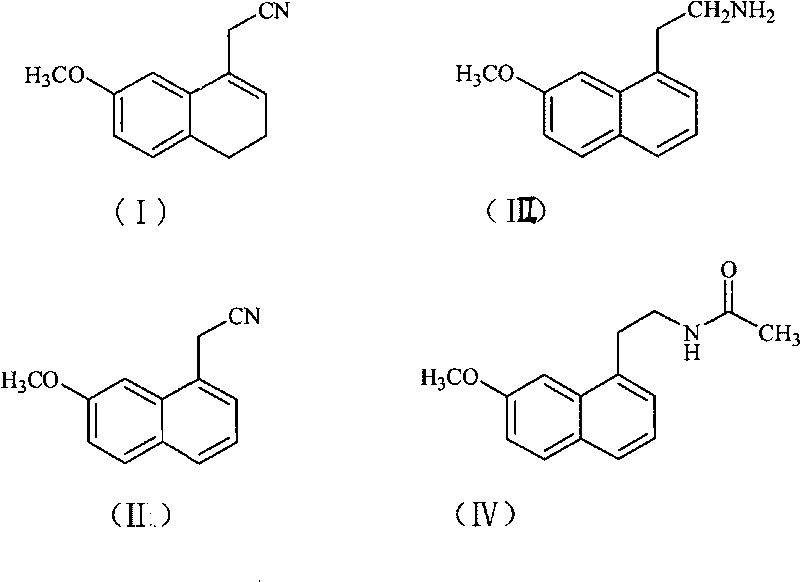
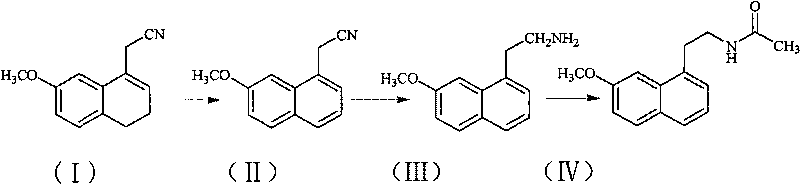
[0030] Preparation of (7-methoxyl-1-naphthyl)acetonitrile:
[0031] 200 g of DDQ and 2 L of chloroform were added to the reaction flask, and a solution of 270 g of (7-methoxy-3,4-dihydro-1-naphthyl)acetonitrile in 0.5 L of chloroform was added dropwise with stirring. After the dropwise addition was completed, the reaction was refluxed. After the reaction was completed, suction filtered, and the filtrate was washed with saturated sodium carbonate solution. The organic phase was dried over anhydrous sodium sulfate, filtered with suction, and the solvent was distilled off from the filtrate under reduced pressure to obtain a brown solid. The solid was stirred in ethanol, filtered with suction, and the filter cake was dried to obtain 251 g of a light yellow solid with a yield of 95%.
[0032] Melting point: 82-84°C
Embodiment 2
[0034] Preparation of 2-(7-methoxy-1-naphthyl)ethylamine:
[0035] Add 193 g of (7-methoxy-1-naphthyl) acetonitrile, 40 g of nickel chloride, and 1 L of ethanol to the reaction flask, and add dropwise a solution of 45 g of sodium borohydride in 0.5 L of ethanol while stirring. After the dropwise addition, react at room temperature. After the reaction was complete, hydrochloric acid was added, and the residue was evaporated to dryness under reduced pressure, dissolved in water, filtered with suction, and the filtrate was washed with ethyl acetate. The aqueous phase was adjusted to pH 9-10 with sodium hydroxide solution, extracted with ethyl acetate, and washed with water until neutral. The organic phase was dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was evaporated to dryness under reduced pressure to obtain 159 g of oil, with a yield of 80%.
Embodiment 3
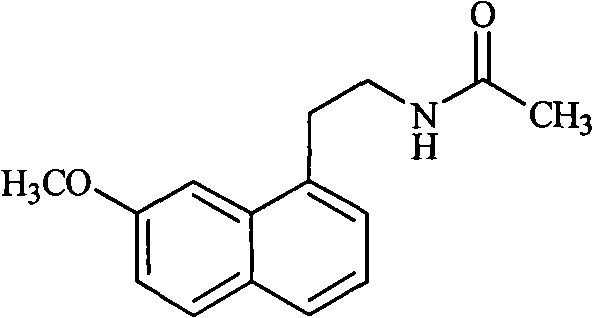
[0037] Preparation of agomelatine (N-[2-(7-methoxy-1-naphthyl) ethyl] acetamide):
[0038] Add 1L of dichloromethane, 126g of 2-(7-methoxy-1-naphthyl)ethylamine to the reaction flask, add 95g of triethylamine under stirring, then dropwise add a mixed solution of 100g of acetyl chloride in 200ml of dichloromethane , room temperature reaction. After the reaction was completed, the solvent was evaporated from the reaction liquid under reduced pressure, water was added, and the crystallization was carried out with stirring. After suction filtration, the filter cake was dried to obtain 148 g of solid, with a yield of 98%.
[0039] Melting point: 105-107°C
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com