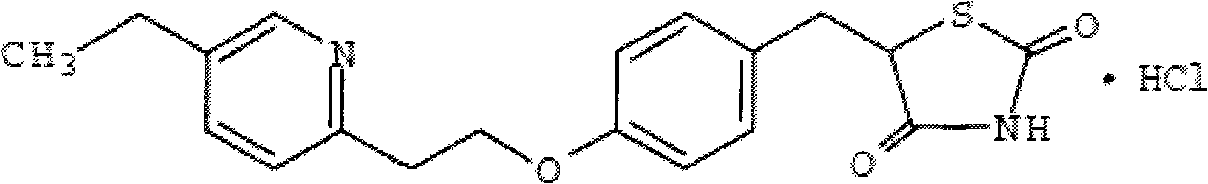
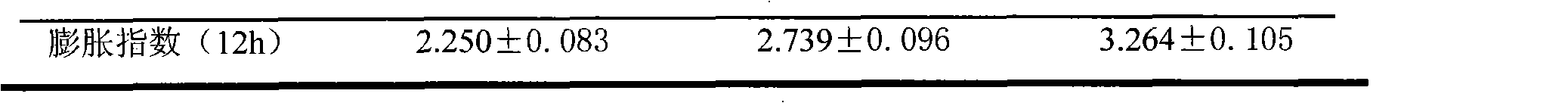Pioglitazone hydrochloride gastric residential sustained release tablet and preparation method thereof
A technology for pioglitazone hydrochloride and retention in the stomach, which is applied to medical formulas, medical preparations containing non-active ingredients, and medical preparations containing active ingredients, etc. It can solve the problems of unstable blood concentration, low bioavailability, and frequent medication problems, to achieve the effect of reducing the number of doses, stable blood concentration, sustained release and absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Pioglitazone hydrochloride and gel forming polymer, foaming agent and pharmaceutical excipients are prepared according to the following weight ratio:
[0027] Pioglitazone Hydrochloride 15mg
[0028] Carbomer 934 60mg
[0029] Sodium bicarbonate 20mg
[0030] Anhydrous citric acid 10mg
[0032] Magnesium stearate 0.5mg
[0033] Polyvinylpyrrolidone K30 6mg
[0034] Separately pulverize and sieve, mix the pioglitazone hydrochloride with the gel-forming polymer, foaming agent and pharmaceutical excipients evenly, pass through the Pharmacopoeia No. 5 sieve, and press the powder into tablets under a suitable pressure.
Embodiment 2
[0036] Pioglitazone hydrochloride and gel forming polymer, foaming agent and pharmaceutical excipients are prepared according to the following weight ratio:
[0037] Pioglitazone Hydrochloride 15mg
[0038] Hypromellose K15M 36mg
[0039] Xanthan Gum 24mg
[0040] Sodium bicarbonate 20mg
[0041] Anhydrous citric acid 10mg
[0043] Magnesium stearate 0.5mg
[0044] Polyvinylpyrrolidone K30 6mg
[0045] Crush and sieve separately, mix pioglitazone hydrochloride, hydroxypropylmethylcellulose K15M, xanthan gum, sodium bicarbonate, anhydrous citric acid and polyvinylpyrrolidone K30 evenly, pass through Pharmacopoeia No. 5 sieve, and dry granulate Add 0.5 mg of magnesium stearate and 0.5 mg of talc powder, mix well and press into tablets under appropriate pressure.
Embodiment 3
[0047] Pioglitazone hydrochloride and gel forming polymer, foaming agent and pharmaceutical excipients are prepared according to the following weight ratio:
[0048] Pioglitazone Hydrochloride 30mg
[0049] Hypromellose K15M 54mg
[0050] Xanthan Gum 36mg
[0051] Sodium bicarbonate 30mg
[0052] Anhydrous citric acid 10mg
[0053] Talc powder 0.8mg
[0054] Magnesium Stearate 1.0mg
[0055] Polyvinylpyrrolidone K30 10mg
[0056] Crush and sieve separately, mix pioglitazone hydrochloride, hydroxypropylmethylcellulose K15M, xanthan gum, sodium bicarbonate, anhydrous citric acid and polyvinylpyrrolidone K30 evenly, pass through Pharmacopoeia No. 5 sieve, and dry granulate Add 1.0 mg of magnesium stearate and 0.8 mg of talc powder, mix well and press into tablets under appropriate pressure.
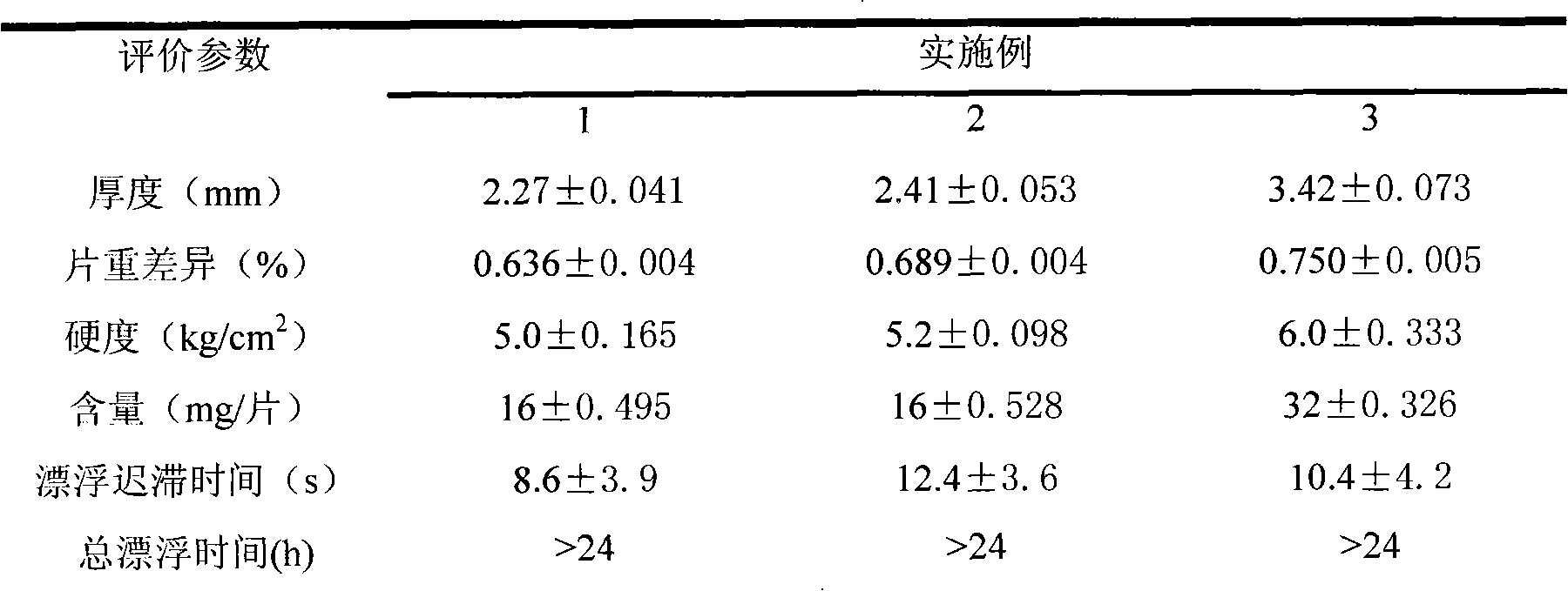
[0057] The managerial index detection is shown in Table 1
[0058] Table 1 Physicochemical Index of Pioglitazone Hydrochloride Sustained-release Tablets Stomach Retention
[0059] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com