Nanometer itraconazole external preparation and preparation method and use thereof
A technology of itraconazole and external preparations, applied in the field of medicine, can solve the problems of difficult absorption, low absorption rate of itraconazole, low skin penetration, etc., achieve bioavailability improvement, avoid liver toxicity, enhance The effect of curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
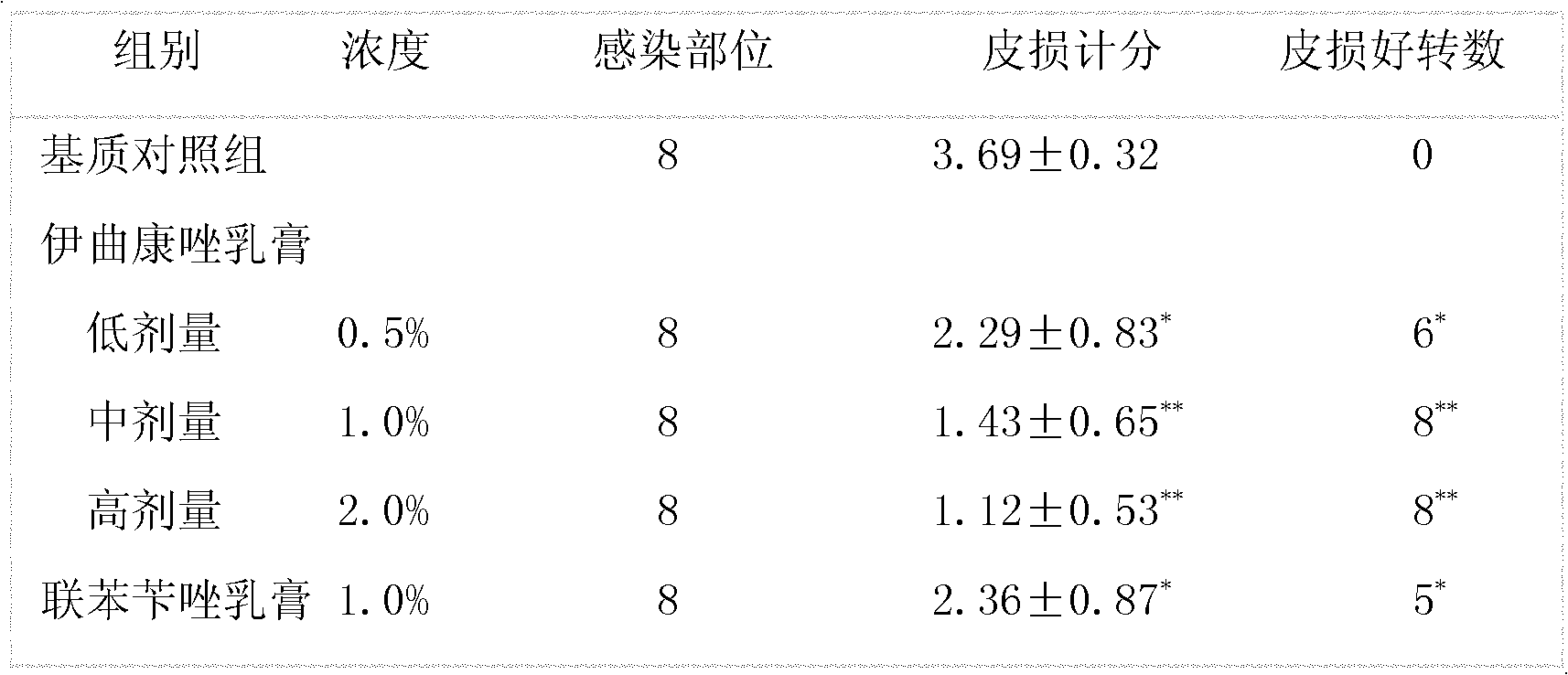
Examples
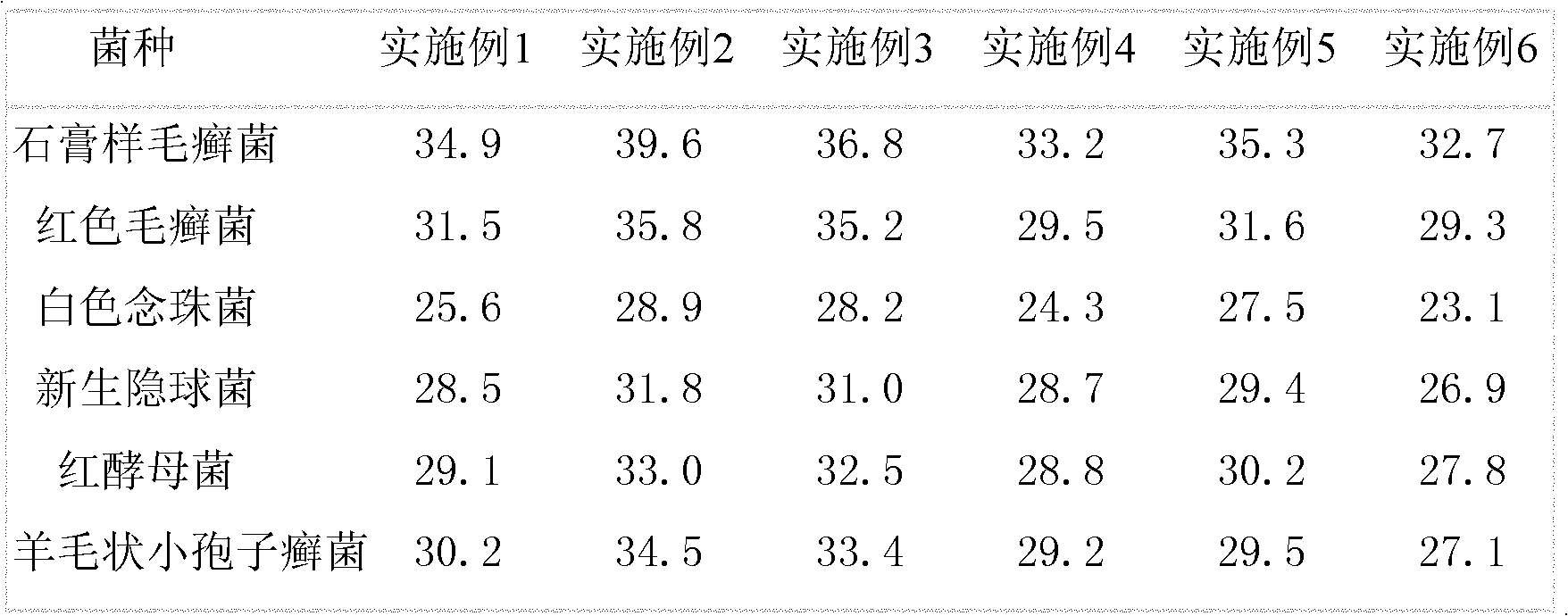
Embodiment 1
[0016] The raw material of itraconazole is pulverized into an average particle size of 150nm by nanotechnology materials, and itraconazole cream is prepared according to the following prescription:
[0017] Itraconazole 10g
[0018] Octadecanol 75g
[0019] Glyceryl monostearate 30g
[0020] White Vaseline 50g
[0021] Liquid paraffin 100g
[0022] Glycerin 75g
[0023] Sodium Lauryl Sulfate 10g
[0024] Methylparaben 1g
[0025] Add water to 1000g
[0026] The raw material of itraconazole is pulverized into an average particle size of 150nm by nanotechnology material, and it is set aside. Take stearyl alcohol, glyceryl monostearate, white petrolatum, and liquid paraffin and heat and melt to form an oil phase, and keep warm at 80-85°C. In addition, the water is heated to 80-85°C, and the prescribed amount of sodium lauryl sulfate and methyl paraben are added to dissolve into the water phase. Pour the water phase into the oil phase, stir while adding, stop heating afte...
Embodiment 2
[0028] The raw material of itraconazole is pulverized into an average particle size of 600nm by nanotechnology materials, and itraconazole cream is prepared according to the following prescription:
[0029] Itraconazole 50g
[0030] Stearic acid 50g
[0031] Glyceryl monostearate 40g
[0032] Octadecanol 55g
[0033] White Vaseline 50g
[0034] Liquid paraffin 55g
[0035] Glycerin 60g
[0036] Tween 80 35g
[0037] Span 60 5g
[0038] EDTA 1g
[0039] Methylparaben 1g
[0040] Add water to 1000g
[0041] The raw material of itraconazole is pulverized into an average particle size of 600nm by nanotechnology material, and it is set aside. Take stearic acid, stearyl alcohol, glyceryl monostearate, white petrolatum, liquid paraffin, and Span 60 and heat and melt to form an oil phase, and keep warm to 80-85°C. In addition, the water is heated to 80-85°C, and then the prescribed amount of Tween 80, methylparaben and EDTA are added to dissolve into the water phase. Pour ...
Embodiment 3
[0043] The itraconazole raw material is pulverized into an average particle size of 150nm by nanotechnology material, and itraconazole ointment is prepared according to the following prescription:
[0044] Itraconazole 50g
[0045] White Vaseline 850g
[0046] Liquid paraffin 50g
[0047] Solid paraffin 50g
[0048] The raw material of itraconazole is pulverized into an average particle size of 150nm by nanotechnology material, and it is set aside. Take white vaseline and solid paraffin and heat to melt, and stir to room temperature. Add the main ingredient into the prescribed amount of liquid paraffin, stir to disperse evenly, pour into the ointment base, and stir evenly. Measure the content, fill and package to obtain itraconazole ointment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com