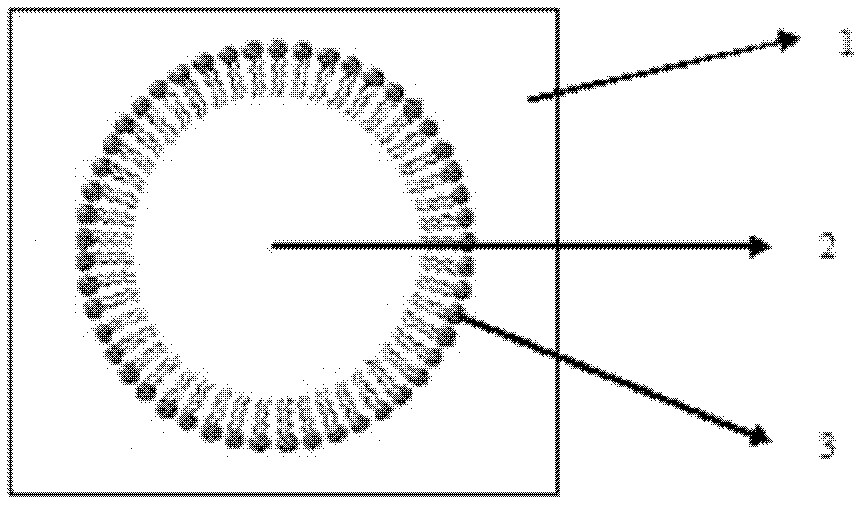
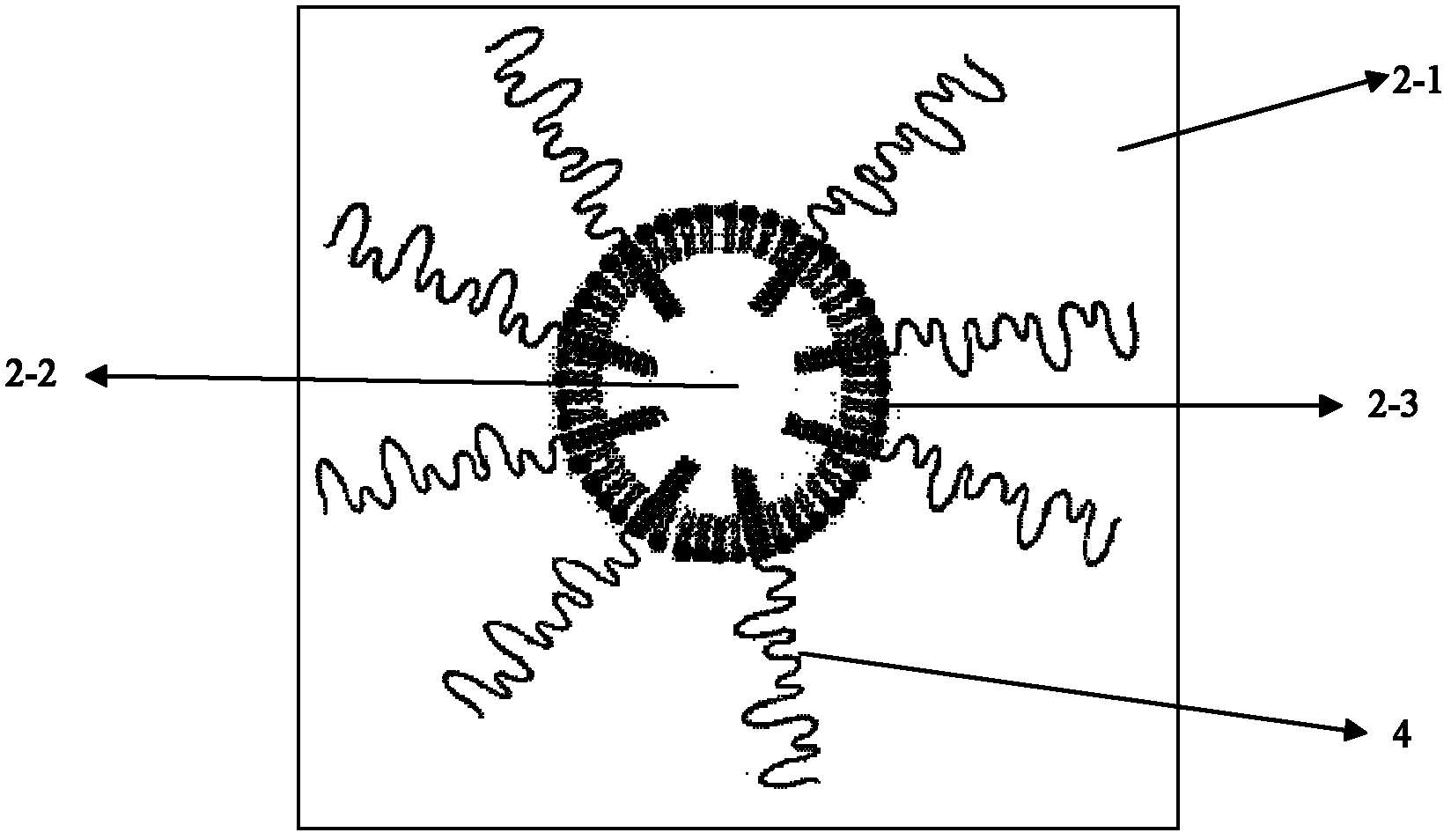
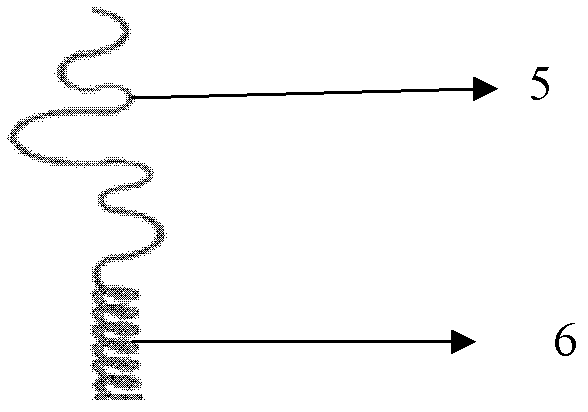
Novel taxol lipid microsphere injection and preparation method thereof
A technology of lipid microspheres and paclitaxel, applied in the field of medicine, can solve problems such as difficult to withstand autoclave sterilization, limited solubility, thermodynamic instability, etc., to improve physical and chemical stability, not easy to precipitate and oxidize, and chemically stable sex good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Preparation of Paclitaxel Lipid Microsphere Injection Containing Amphiphilic Polyamino Acids
[0062] [Prescription 1] Specification: 30mg / 50ml
[0063] Paclitaxel 0.06%
[0064] Medium Chain Triglycerides (MCT) 15%
[0065] PEG 5000 - Racemic polyleucine 3000 0.1%
[0066] Soy Lecithin 3%
[0067] Tween 80 0.1%
[0068] Poloxamer 188 0.3%
[0069] Glycerin 2.5%
[0070] Edetate Disodium 0.02%
[0071] Add water for injection to 100%
[0072] Preparation:
[0073] (1) Dissolve the prescribed amount of paclitaxel and soybean lecithin in absolute ethanol under heating and stirring at 75°C, and evaporate the absolute ethanol to dryness. Add the prescribed amount of medium-chain fatty acid triesters and oleic acid, heat and stir at 75°C, continue to evaporate ethanol under nitrogen flow, and obtain a clear medicated oil phase for future use.
[0074] (2) Change the prescription amount to PEG 5000 - Racemic polyleucine 3000 , polysorbate-...
Embodiment 2
[0207] Prescription of paclitaxel lipid microsphere injection containing amphiphilic polyamino acid (prescription 4)
[0208] 6-month acceleration and long-term stability investigation
[0209] Since this product is a thermodynamically unstable system, it is a heat-sensitive dosage form. According to the instructions of the Chinese Pharmacopoeia 2005 edition of the guidelines for the stability test of raw materials and pharmaceutical preparations: the accelerated test of heat-sensitive drugs should be placed at 25±2°C for 6 months; at 6±2°C for 12 months, the investigation Changes in physical and chemical stability, the experiment has been carried out for 6 months, and the results of the 6-month investigation are given.
[0210] Table 1 25±2℃ Accelerated 6-month test results
[0211]
[0212] Table 2 Result of long-term retention sample test at 6±2°C
[0213]
[0214] After 6 months of accelerated testing, all quality indicators are within the qualified range. Th...
Embodiment 3
[0216] Study on Plasma Pharmacokinetics of Paclitaxel Lipid Microsphere Injection of the Present Invention in Rats
[0217] Dosing regimen:
[0218] 36 rats were randomly divided into 6 groups, 6 rats in each group, fasted overnight before the experiment. The commercially available paclitaxel injection and the paclitaxel lipid microsphere injection containing amphiphilic polyamino acid of the present invention were injected into the right posterior femoral vein respectively, and the grouping and administration conditions are as follows:
[0219]
[0220] Before administration (0 o'clock), 0.3 mL of orbital blood was collected at 5 min, 15 min, 30 min, 60 min, 90 min, 2 h, 4 h, 6 h, 8 h, and 12 h, and placed in pre-heparinized 1.5 Centrifuge at 4000 rpm for 15 min in mL conical-bottomed centrifuge tubes, accurately draw 100 μL of the upper layer of plasma, put it in another clean conical-bottomed centrifuge tube, store in the refrigerator, add 25.4 μg·mL -1 20 μL of inte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com