Method for synthesizing ethyldivaricatinate and application of ethyldivaricatinate
A technology of ethyl arachidonic acid and a synthesis method is applied in the directions of carboxylate preparation, chemical instruments and methods, applications, etc., and can solve the problems of difficult industrialized mass production, low product yield, expensive bromination reagents, etc. Less oral irritation, avoiding high temperature conditions, and good smoke coordination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
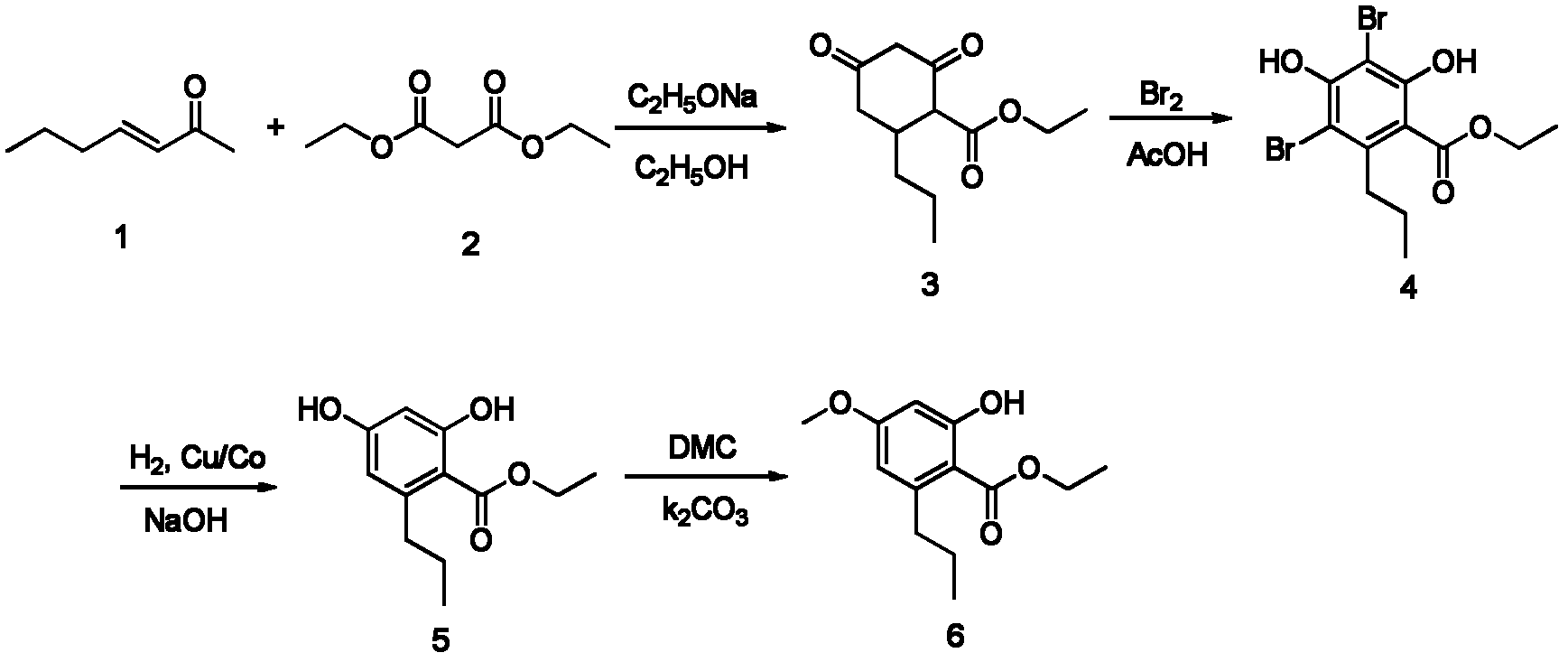
[0040] Organic intermediate (3): the synthesis of 2,4-dioxo-6-propyl-cyclohexane ethyl carboxylate comprises the following steps:
[0041] In a 50ml one-necked flask, weigh 0.71g (10.5mmol) of sodium ethoxide, add 15ml of absolute ethanol solution, and stir until the sodium ethoxide is completely dissolved. Add 1.6g (10mmol) of diethyl malonate (2) and 1.12g (10mmol) of 3-hepten-2-one (1) in sequence, install a condensing reflux and drying device, and heat up to 40°C , reacted for 5 hours. As the reaction progressed, the color of the solution turned yellow, accompanied by the precipitation of a large amount of light yellow powdery solid.
[0042] After the reaction, acidify with hydrochloric acid, extract with dichloromethane, and remove the dichloromethane by rotary evaporation to obtain 1.88 g of ethyl 2,4-dioxo-6-propyl-cyclohexanecarboxylate in a yellow oily liquid, with a yield of 83 %.
[0043] MS(EI): m / z(%)=226(M + , 10), 183(75), 155(20), 143(25), 137(78), 113(40)...
Embodiment 2
[0045] Organic intermediate (4): 3,5-dibromo-2, the synthesis of 4-dihydroxyl-6-propyl benzoic acid ethyl ester, comprises the following steps:
[0046] In a 50ml single-necked flask, add 1.13g (5mmol) of 2,4-dioxo-6-propyl-cyclohexylcarboxylate ethyl ester prepared in Example 1, then add 15ml of acetic acid therein, and stir Make it dissolve evenly; then measure 0.77ml (15mmol) of liquid bromine and add it dropwise to the reaction solution. After the dropwise addition, react at 25°C for 15h.
[0047] After the reaction, the reaction solution was poured into 50ml of water, and a small amount of anhydrous sodium sulfite was added to remove excess liquid bromine to remove the color of the solution. After stirring for a while, a large amount of yellow solid was formed, which was filtered, washed with water, and dried to obtain 1.69 g of yellow solid with a yield of 88%. The obtained yellow solid was separated and purified by silica gel column chromatography, eluted with a mixed ...
Embodiment 3
[0050] Organic intermediate (5): the synthesis of ethyl 2,4-dihydroxy-6-propylbenzoate comprises the following steps:
[0051] 1.14g (3mmol) 3,5-dibromo-2,4-dihydroxyl-6-propyl benzoic acid ethyl ester that embodiment 2 makes is dropped in the single port reaction flask of 50ml, adds 7.5ml 2N NaOH solution, After stirring to make it dissolve, add 0.17g of 10% Cu / Co catalyst, and stir to make the catalyst evenly distributed. Install a three-way valve in the reaction flask, put a hydrogen-filled balloon on the three-way valve, adjust the valve position of the three-way valve, vacuumize the air in the reaction flask, and then adjust the valve to introduce the hydrogen in the balloon into the reaction flask , repeat 1 time. Under the condition of 25°C, the reaction was stirred for 24 hours, and hydrogen was replenished twice by balloon on the way.
[0052] After the reaction, the Cu / Co in the reaction solution was removed with a sand core funnel to obtain a clear brown reaction ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com