Cationic polymer as well as preparation method and application thereof
A cationic polymer and polymer technology, applied in the direction of using vectors to introduce foreign genetic material, recombinant DNA technology, etc., can solve problems such as transfection failure and cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1 contains the synthesis of the alkynylated triethylenetetramine monomer (numbering A) of Boc
[0073] Triethylenetetramine (1.46g, 10mmol) was dissolved in DCM (50mL) solution, and after cooling in an ice bath, ethyl trifluoroacetate (2.95g, 21mmol) was added dropwise, and stirred for 1h under ice bath. After the temperature of the reaction solution rose to room temperature and then stirred for 1 h, Boc dissolved in an appropriate amount of DCM was added dropwise. 2 O (5.68g, 26mmol) solution and TEA (2.63g, 26mmol) solution were stirred at room temperature overnight. with saturated NaHCO 3 Washed with saturated brine, anhydrous Na 2 SO 4 dry. After concentration, it was recrystallized with DCM / n-hexane system.
[0074] The above-mentioned recrystallized product (2.00g) was mixed with K 2 CO 3 (1.80 g) was refluxed in methanol / water (volume ratio 20:1) system for 4 h, methanol was spin-dried, and the residue was extracted with DCM (3×100 mL). The com...
Embodiment 2
[0077] Synthesis of embodiment 2 azide diethylene glycol monomer (numbering 1)
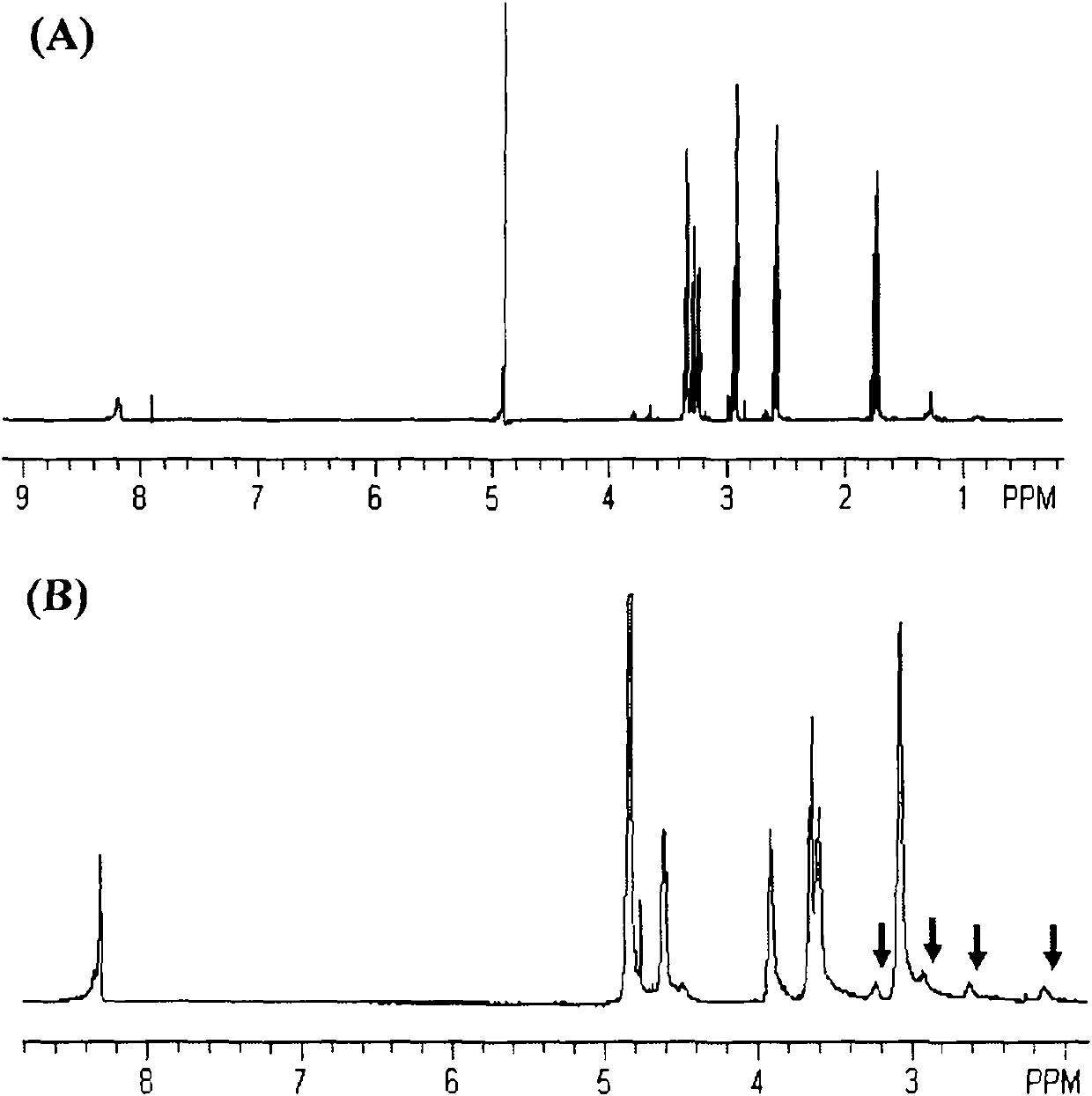
[0078] Diethylene glycol (2.12 g, 20 mmol) was dissolved in DCM (250 mL) and cooled in an ice bath. Add p-toluenesulfonyl chloride (7.62g, 42mmol), TEA (4.25g, 42mmol) and 4-dimethylaminopyridine DMAP (0.12g, 0.1mmol) in sequence, stir in ice bath for 1h, and then stir at room temperature for 12h. The reaction solution was sequentially washed with saturated NaHCO3 (2×300mL) and saturated brine (300mL) for washing, anhydrous Na 2 SO 4 dry. After concentration, it was recrystallized with DCM / n-hexane system. Dissolve the recrystallized product (4.1 g, 10 mmol) in acetone, add sodium azide (1.9 g, 30 mmol), and reflux for 48 h. The solvent was concentrated, separated by silica gel column chromatography, and eluted with petroleum ether / ethyl acetate (volume ratio 2:1) to obtain monomer 1 ( 1 HNMR (DMSO-d 6 ): 2.87(s, 4H), 2.70(s, 4H)). The structural formula of monomer 1 is as follows:
[0079...
Embodiment 3
[0080] Synthesis of embodiment 3 azide-containing disulfide bond-containing monomer (numbering b)
[0081] Dissolve propylamine chloride (1.30 g, 10 mmol) in water, add sodium azide (1.95 g, 30 mmol), and react at 80° C. for 15 h. After removing most of the water under reduced pressure, the reaction solution was cooled in an ice bath, and sodium hydroxide (4 g) was added to neutralize the hydrochloric acid. The aqueous solution was extracted with diethyl ether and washed with diethyl ether (3×100 mL), the combined organic phases were washed with anhydrous K 2 CO 3 Drying; Concentrate to obtain azidopropylamine;
[0082] Dissolve 3,3'-dithiodipropionic acid (2.10g, 10mmol) in water (100mL), and add 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride respectively (EDC, 4.34g, 22mmol) and N-hydroxysuccinimide (NHS, 2.53g, 22mmol), reacted for 2h, added azidopropylamine (2.20g, 22mmol), and continued to react at room temperature for 24h. The reaction solution was extract...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com