Paclitaxel bonded amphipathic aliphatic polyester-b-polyphosphate high polymer and preparation thereof
An aliphatic polyester and polyphosphate technology, which is applied in the direction of artificially synthesized active ingredients of polymeric materials, drug combinations, anti-tumor drugs, etc., can solve the problem of no anti-cancer drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
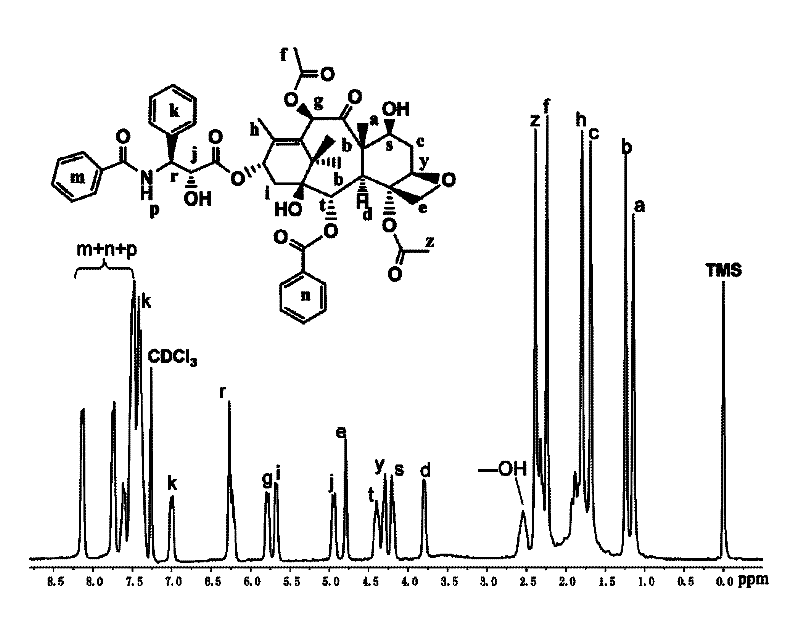
[0065] Example 1: Preparation of a polymer drug bonded by paclitaxel-polyε-caprolactone
[0066] Put the vial, glass syringe, needle and glass stopper with the stir bar in an oven at 120°C for 12 hours to dry, then take out the syringe and needle and put it in a desiccator to cool, plug the vial with a ground glass stopper and connect it Use an oil pump to cool to room temperature on the double-row tube, then fill with high-purity argon, and vent three times, and finally fill with argon, add white paclitaxel powder (0.20g, 0.23mmol) and Stannous octoate (0.047g, 0.12mmol) viscous liquid. Exhaust air twice, use a dry syringe to extract the solvent o-dichlorobenzene after argon exchange, add it to the branch bottle, stir to dissolve until the system is clear and transparent. Then use a dry syringe to add ε-caprolactone monomer (1.33 g, 0.012 mol), and the molar ratio of the amount to the initiator paclitaxel is 40-60:1. Put the reaction flask into the set 90-110°C oil bath, and r...
Embodiment 2
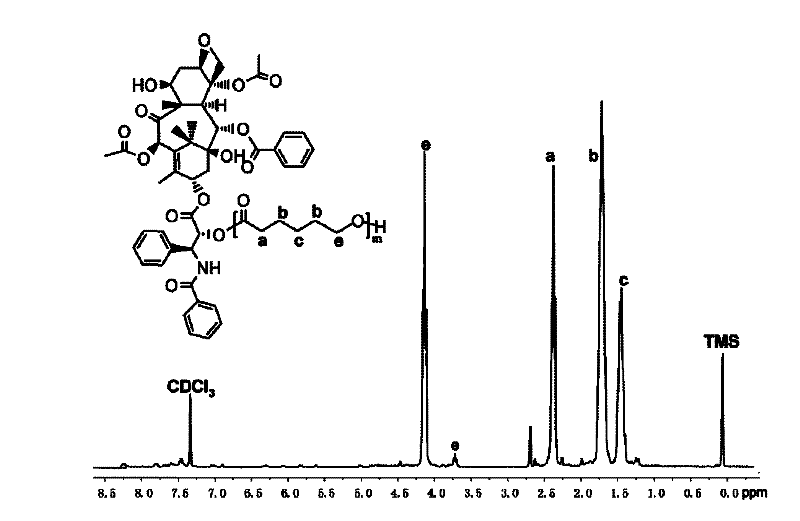
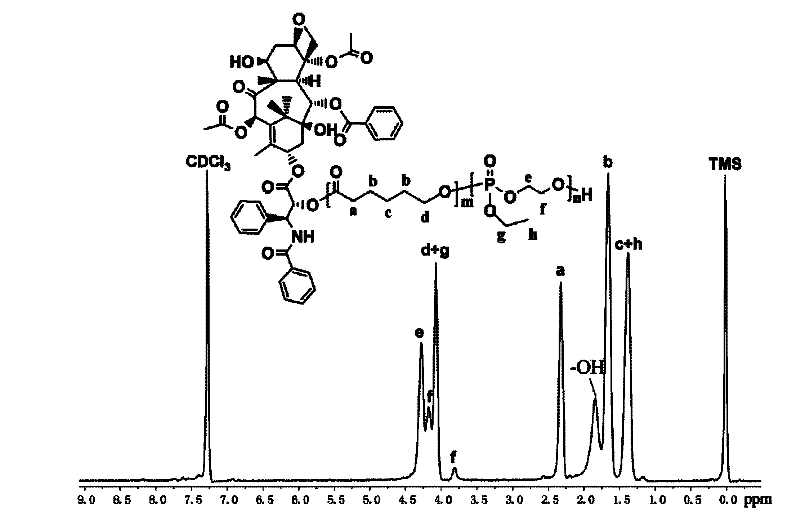
[0067] Example 2: Preparation of paclitaxel-polyε-caprolactone-polyphosphate bonded polymer drug (PTX-PCL-b-PEEP)
[0068] Place the vial, glass syringe, needle and ground glass stopper with the stir bar in an oven at 120°C for 12 hours to dry, remove the syringe and needle into a desiccator to cool, and stopper the vial bottle with the ground glass stopper Then connect it to the double-row pipe and use an oil pump to cool to room temperature, then fill with high-purity argon, and pump for three times, and finally fill with argon, add the number average molecular weight of about 4800g / mmol paclitaxel under the condition of argon Bonded polyε-caprolactone (PTX-PCL 35 ) Macromolecular initiator (0.58g, 0.12mmol) and stannous octoate (0.024g, 0.06mmol) viscous liquid. Exhaust air twice, use a dry syringe to extract tetrahydrofuran and add the solvent tetrahydrofuran to the branch vial, stir to dissolve until the system is clear and transparent. Then use a syringe to add phosphate m...
Embodiment 3
[0069] Example 3: Preparation of polymer micelles containing anticancer drugs by film forming method
[0070] Take 5mg sample PTX-PCL in a round bottom flask 35 -b-PEEP 16 Stir in 5 mL of tetrahydrofuran to fully dissolve, and then completely remove the tetrahydrofuran by rotary evaporation, then add 5 mL of phosphate buffer solution with a pH of 7.4, and stir overnight. Its morphology and micelle size were observed by transmission electron microscope, see Figure 5 .
[0071] The above PTX-PCL 35 -b-PEEP 16 It self-assembles in phosphate buffer solution to form micelles with hydrophobic drugs and lipophilic aliphatic polyester as the core and water-soluble polyphosphate as the shell. On the one hand, it can improve the strong hydrophobicity of the paclitaxel drug, thereby avoiding the rejection reaction in the process of drug delivery in the body; on the other hand, through the degradation of polyester, slow release of the drug, avoiding the sharp increase in drug concentration to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com