Acetaminophen-cyclophosphamide anti-tumor drug and preparation method thereof
A technology of paracetamol and antitumor drugs, applied in the field of medicine, can solve the problems of affecting clinical use, poor selectivity, etc., and achieve the effects of good analgesic effect, good cross-linking effect and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the synthesis of compound 5
[0022] Step 1: Preparation of Compound 3
[0023] Bisdichloroethylamine hydrochloride (1) (20g, 0.112mol) and 52mL (0.60mol) of freshly distilled POCl 3 (2) Place in a 200mL round bottom flask, reflux at 110°C for 20h until the solid bisdichloroethylamine hydrochloride disappears. Excess POCl was removed under reduced pressure 3 , the resulting solid residue was dissolved in ethyl acetate, filtered to remove insoluble matter, concentrated ethyl acetate filtrate, and the filtrate was recrystallized with acetone:petroleum ether (v / v=1:5) to obtain colorless crystals, namely phosphoryl nitrogen A total of 18.0 g of mustard dichloride 3 was obtained, and the yield was 61%. The structure of the product was determined by melting point, IR, NMR, MS and elemental analysis.
[0024] Step 2: Preparation of Compound 5
[0025] Paracetamol (4) (151 mg, 1 mmol) was dissolved in 20 mL of methanol, and triethylamine (185 mL, 1.34 mmol) w...
Embodiment 2
[0026] Embodiment 2: Anti-tumor experiment in vitro
[0027] The compound 5 of the present invention was selected to test its cytotoxicity to cell lines HepG-2, A549 and HL-60 by MTT method.
[0028] 1. Compound 5 was formulated into different concentrations in RPMI-1640 culture medium and stored at 4°C.
[0029] 2. Cancer cells were cultured in RPMI-1640 medium containing 10% calf serum, 100kU / L penicillin and 100mg / L streptomycin in 5% CO 2 Cultured at constant temperature in a saturated humidity cell incubator.
[0030] 3. Take the cancer cells in the logarithmic growth phase, add 5.0×10 4 cells / mL cell suspension 100 μL.
[0031] 4. Set blank and cyclophosphamide (CP), 5-fluorouracil (5-Fu) positive control blank at the same time, add the same volume of culture medium to the blank group, add 4 μmol / L to the positive control group, set eight parallel wells for each concentration .
[0032] 5. After continuing to culture for 48 hours, add MTT (5 g / L, 20 μL) to each well...
Embodiment 3
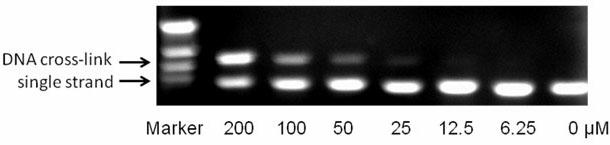
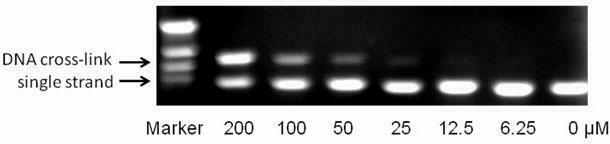
[0038] Example 3: Cross-linking of Drugs and DNA
[0039] Compound 5 of the present invention was selected to detect its in vitro cross-linking activity on plasmid pBR322DNA, using alkaline gel electrophoresis.
[0040] 1. Prepare linear pBR322DNA. Prepare a mixture of the following systems (total 200 μL): supercoiled pBR322DNA (10 μL, 10 μg), restriction enzyme EcoR I (4 μL, 40-80u), 10 times EcoR I buffer solution (20 μL), acetylated BSA ( 20μL, 1mg / mL), secondary water (146μL). The above mixture was incubated in a constant temperature water tank at 37°C for 3 hours, then sodium acetate (20 μL, 3M) and glacial ethanol (440 μL) were added, and allowed to stand overnight at -20°C. Remove the mixture, centrifuge at 16,000 rpm for 15 minutes, pour off the ethanol, and invert for several minutes to evaporate residual ethanol. Add 30 μL of secondary water for later use. The concentration is about 0.25μg / μL.
[0041] 2. Add 2 μL (0.25 μg / μL) of linearized pBR322DNA into a 0.2 ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap