Catalyst for methanation of CO and H2, and preparation method thereof
A catalyst and methanation technology, applied in the field of CO and H2 methanation catalysts and their preparation, can solve the problems of easy agglomeration, catalyst sintering, and narrow catalyst activity temperature range, and achieve easy large-scale production and strong anti-sintering performance. , the shape regular and controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
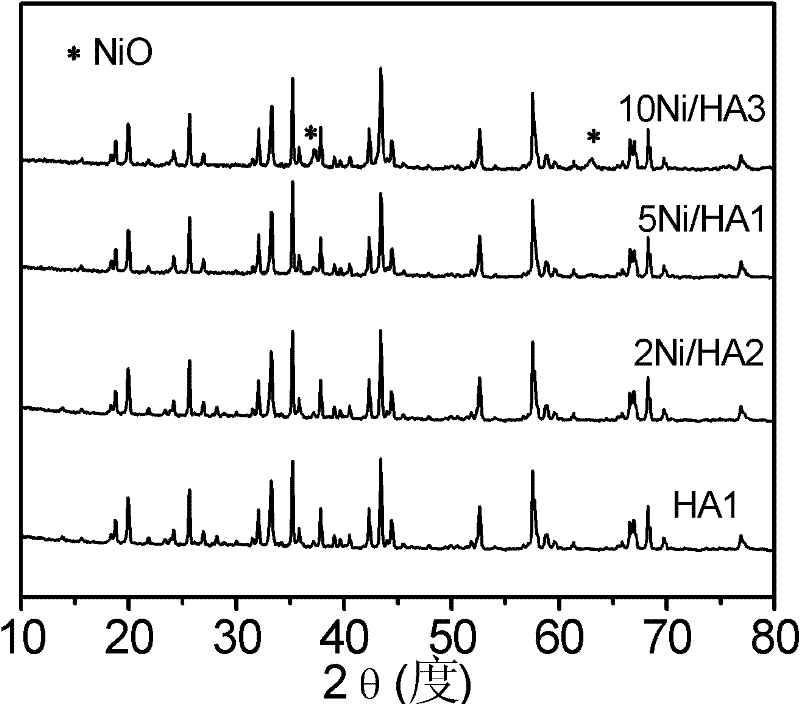
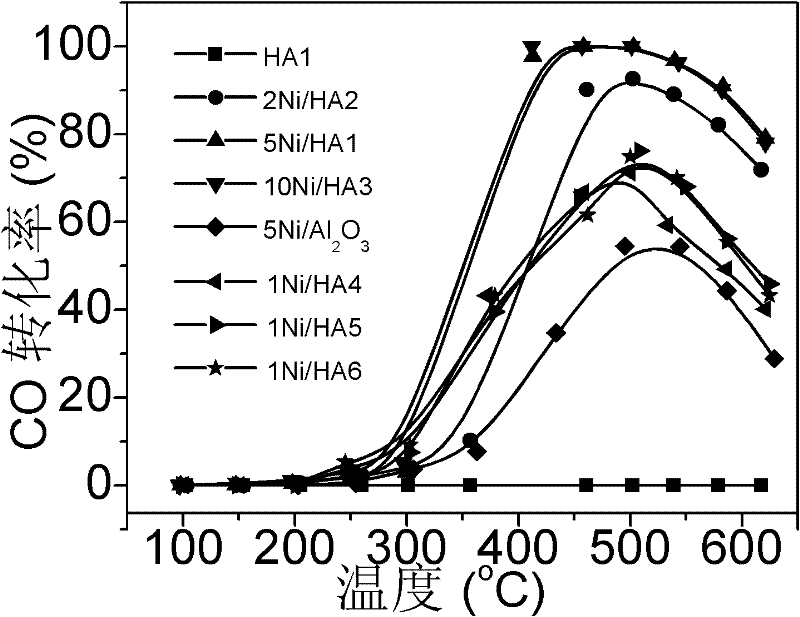
Image
Examples
Embodiment 1
[0062] Preparation of catalyst carrier (barium hexaaluminate carrier): Dissolve 4g of surfactant polyoxypropylene polyoxyethylene copolymer (P123) in 20g of ethanol, and after P123 is completely dissolved, heat the solution to 40°C to form 0.27mol / L solution, and then add 60mL of a solution with a concentration of 2mol / L aluminum nitrate, stir and mix evenly. In addition, take 2g BaCO 3 Dissolve in 10mL 5mol / L acetic acid aqueous solution, wherein the concentration of barium ion is 1mol / L, the molar ratio of metal precursor and acetic acid is 1:5, add 4mol / L nitric acid to adjust the pH to 1, add the barium salt solution In the above-mentioned mixed solution containing P123 and aluminum salt, the molar ratio of alkaline earth metal and aluminum in the obtained solution is 1:12, the obtained mixture is evaporated in an oven at 40°C to form xerogel, and the obtained xerogel is heated at 100°C After drying for 6 hours, heat-treat the dried xerogel at 1200°C for 2 hours in a nit...
Embodiment 2
[0069] Preparation of catalyst carrier (barium hexaaluminate carrier): Dissolve 4g of surfactant cetyltrimethylammonium bromide (CTAB) in 20g of ethanol, and after CTAB is completely dissolved, heat the solution to 40°C to form 0.5 mol / L solution, and then add 60mL of a solution with a concentration of 2mol / L aluminum nitrate, stir and mix evenly. In addition, take 3g BaCO 3 Dissolve in 15mL 5mol / L acetic acid aqueous solution, wherein the concentration of barium ion is 1mol / L, the molar ratio of alkaline earth metal precursor and acetic acid is 1:3, add the prepared 4mol / L nitric acid, adjust the pH to 1, and The barium salt solution is added to the above-mentioned mixed solution containing CTAB and aluminum salt, the molar ratio of alkaline earth metal and aluminum in the obtained solution is 1:8, and the obtained mixture is evaporated in an oven at 40° C. to form xerogel. The xerogel is first calcined in argon at 1200°C for 2 hours to carbonize CTAB in situ and form hexaal...
Embodiment 3
[0073] Preparation of catalyst carrier (barium hexaaluminate carrier): Dissolve 4g of surfactant P123 in 20g of ethanol, heat the solution to 40°C to form a 0.27mol / L solution after P123 is completely dissolved, then add 60mL of 2mol / L aluminum nitrate solution, stir quickly and mix well. Also, take 4g BaCO 3 Dissolve in 20 mL of 5 mol / L (mass ratio) acetic acid aqueous solution, wherein the concentration of barium ions is 1 mol / L, the molar ratio of metal precursor and acetic acid is 1:5, add the prepared 4 mol / L nitric acid, and adjust the pH to 1 , adding the barium salt solution to the above-mentioned mixed solution containing P123 and aluminum salt, the molar ratio of alkaline earth metal and aluminum in the obtained solution is 1:6, and the obtained mixture is evaporated in an oven at 40° C. to form xerogel. The xerogel is firstly heat-treated in helium at 1200°C for 2 hours to carbonize P123 in situ and form a hexaaluminate crystal phase, and then bake it in an air at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com